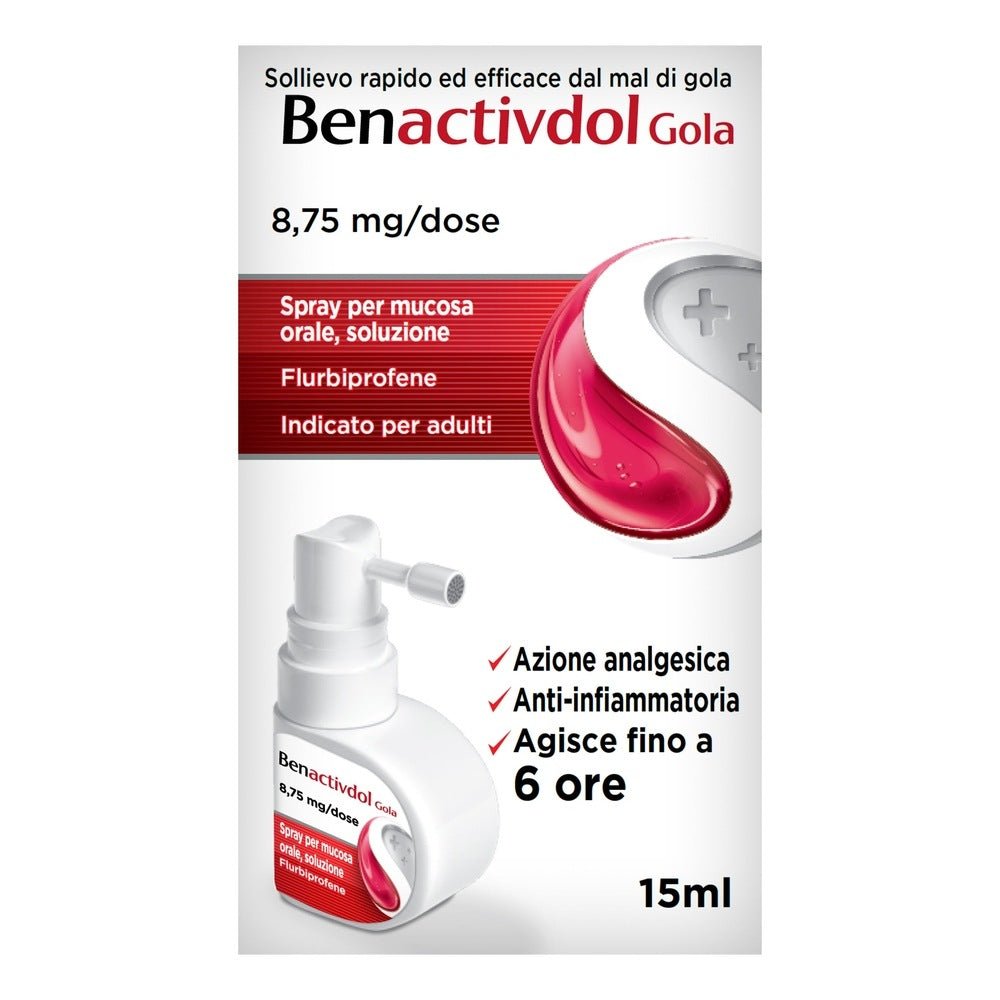
RECKITT BENCKISER H.(IT.) SpA
Benactivdol Throat 8.75mg/dose spray 15ml
Benactivdol Throat 8.75mg/dose spray 15ml
Regular price
€13,90
Regular price
€13,90
Sale price
€13,90
Unit price
per
Tax included.
Shipping calculated at checkout.

Pickup available at Farmacia Tili
Usually ready in 24 hours
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
EAN
EAN
043050018
MINSAN
MINSAN
043050018
Benactivdol Gola 8.75mg/dose spray 15ml is an effective treatment for acute throat pain in adults. This oromucosal spray contains Flurbiprofen , an active ingredient known for its anti-inflammatory and analgesic properties. Each dose, consisting of three sprays, provides 8.75 mg of Flurbiprofen, ensuring rapid relief from the symptoms of sore throat. The formulation is enriched with mint and cherry flavors , making the application more pleasant. Benactivdol Gola is designed for practical and targeted use, offering a localized action directly on the affected area. This product is ideal for those looking for a rapid and specific remedy for sore throat, thanks to its combination of effectiveness and ease of use.
Reporting of suspected adverse reactions Reporting suspected adverse reactions that occur after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system at https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse.
ACTIVE INGREDIENTS
Active ingredients contained in Benactiv Gola 0.25% spray for oral mucosa 15ml - What is the active ingredient of Benactiv Gola 0.25% spray for oral mucosa 15ml?
BENACTIV GOLA Mouthwash 100 ml of mouthwash contain: active ingredient: flurbiprofen 250 mg Excipients with known effects: hydrogenated castor oil-40-polyoxyethylene, methyl parahydroxybenzoate, propyl parahydroxybenzoate, mint essence (containing d-limonene). BENACTIV GOLA Oral mucosa spray 100 ml of solution contain: active ingredient: flurbiprofen 250 mg Excipients with known effects: hydrogenated castor oil-40-polyoxyethylene, methyl parahydroxybenzoate, propyl parahydroxybenzoate, mint essence (containing d-limonene). BENACTIV THROAT Lemon and Honey Flavour Lozenges One lozenge contains: active ingredient: flurbiprofen 8.75 mg Excipients with known effects: liquid glucose (containing sulphites and wheat starch), liquid sucrose, honey, lemon flavouring and levomenthol (containing butylated hydroxyanisole, citral, citronellol, d-limonene, farnesol, geraniol, linalool). BENACTIV THROAT Sugar Free Lozenges Orange Flavour One lozenge contains: active ingredient: flurbiprofen 8.75 mg Excipients with known effects: liquid maltitol (E965), isomalt (E953), orange flavouring and levomenthol (containing citral, citronellol, d-limonene, geraniol, linalool). For the full list of excipients, see section 6.1.EXCIPIENTS
Composition of Benactiv Gola 0.25% spray for oral mucosa 15ml - What does Benactiv Gola 0.25% spray for oral mucosa 15ml contain?
BENACTIV THROAT Mouthwash Glycerol, ethanol (96 percent), sorbitol 70, polyoxyethylene-40-hydrogenated castor oil, sodium hydroxide, sodium saccharin, methyl parahydroxybenzoate, propyl parahydroxybenzoate, mint essence (containing d-limonene), patent blue V (E131), purified water. BENACTIV THROAT Oral mucosa spray Glycerol, ethanol (96 percent), sorbitol 70, polyoxyethylene-40-hydrogenated castor oil, sodium hydroxide, sodium saccharin, methyl parahydroxybenzoate, propyl parahydroxybenzoate, mint essence (containing d-limonene), patent blue V (E131), purified water. BENACTIV THROAT Lemon and Honey flavoured lozenges Liquid sucrose, liquid glucose (containing sulphites and wheat starch), macrogol 300, potassium hydroxide, lemon and levomenthol flavouring (containing butylated hydroxyanisole, citral, citronellol, d-limonene, farnesol, geraniol, linalool), honey. BENACTIV THROAT Sugar-free lozenges Orange flavoured Macrogol 300, potassium hydroxide, orange and levomenthol flavouring (containing citral, citronellol, d-limonene, geraniol, linalool), acesulfame potassium (E950), liquid maltitol (E965), isomalt (E953).DIRECTIONS
Therapeutic indications Benactiv Gola 0.25% spray for oral mucosa 15ml - Why is Benactiv Gola 0.25% spray for oral mucosa 15ml used? What is it used for?
BENACTIV THROAT Mouthwash BENACTIV THROAT Spray for oral mucosa Symptomatic treatment of irritative-inflammatory conditions also associated with pain in the oropharyngeal cavity (e.g. gingivitis, stomatitis, pharyngitis), also as a consequence of conservative or extractive dental therapy. BENACTIV THROAT Lemon and Honey flavour lozenges BENACTIV THROAT Sugar-free lozenges Orange flavour Symptomatic treatment of irritative-inflammatory conditions also associated with pain in the oropharyngeal cavity (e.g. gingivitis, stomatitis, pharyngitis).CONTRAINDICATIONS SIDE EFFECTS
Contraindications Benactiv Gola 0.25% spray for oral mucosa 15ml - When should Benactiv Gola 0.25% spray for oral mucosa 15ml not be used?
Do not use the medicine in children under 12 years of age. Flurbiprofen is contraindicated in patients with known hypersensitivity to flurbiprofen or to any of the excipients listed in section 6.1. Patients who have previously shown hypersensitivity reactions (e.g. asthma, urticaria, allergy, rhinitis, angioedema, bronchospasm) to ibuprofen, acetylsalicylic acid (aspirin) or other nonsteroidal anti-inflammatory drugs (NSAIDs). Flurbiprofen is also contraindicated in patients with a history of gastrointestinal bleeding or perforation related to previous NSAID treatment. Flurbiprofen should not be taken by patients with active or history of ulcerative colitis, Crohn's disease, recurrent peptic ulcer or gastrointestinal bleeding (defined as two or more distinct episodes of proven ulceration or bleeding). Flurbiprofen is contraindicated in patients with severe heart failure, severe hepatic failure and renal failure (see section 4.4). Third trimester of pregnancy.DOSAGE
Quantity and method of taking Benactiv Gola 0.25% spray for oral mucosa 15ml - How to take Benactiv Gola 0.25% spray for oral mucosa 15ml?
Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms (see section 4.4). BENACTIV GOLA Mouthwash Dosage Adults : 2-3 rinses or gargles per day with 10 ml (1 measuring spoon) of mouthwash. Paediatric population Children over 12 years of age: as for adults. Children under 12 years of age: do not administer to children under 12 years of age (see section 4.3). Special populations Elderly : the clinical data currently available are limited, therefore no recommendation on posology can be made. The elderly are at greater risk of serious consequences in case of adverse reactions (see section 4.4). Patients with hepatic insufficiency : a dosage reduction is not necessary in patients with mild to moderate hepatic insufficiency. Flurbiprofen is contraindicated in patients with severe hepatic insufficiency (see section 4.3). Patients with renal insufficiency : no dosage reduction is necessary in patients with mild to moderate renal insufficiency. Flurbiprofen is contraindicated in patients with severe hepatic insufficiency (see section 4.3). Method of administration For oropharyngeal use. Rinse or keep in the mouth during gargling for up to 1 minute. Do not swallow. The mouthwash can be used pure or diluted in half a glass of water. BENACTIV GOLA Spray for oral mucosa Dosage Adults : apply one dose (2 sprays) 3 times a day, directed directly at the affected area. Each spray delivers 0.2 ml of solution, equivalent to 0.5 mg of active ingredient. Paediatric population Children over 12 years of age: as for adults. Children under 12 years of age: do not administer to children under 12 years of age (see section 4.3). Special populations Elderly : The clinical data currently available are limited, therefore no recommendation on posology can be made. Elderly are at increased risk of serious consequences in case of adverse reactions (see section 4.4). Patients with hepatic impairment : no dosage reduction is necessary in patients with mild to moderate hepatic impairment. Flurbiprofen is contraindicated in patients with severe hepatic impairment (see section 4.3). Patients with renal impairment : no dosage reduction is necessary in patients with mild to moderate renal impairment. Flurbiprofen is contraindicated in patients with severe hepatic impairment (see section 4.3). Method of administration For oropharyngeal use. Aim the dispenser towards the back of the throat and spray on the affected area. BENACTIV THROAT Lemon and Honey flavour lozenges BENACTIV THROAT Sugar-free lozenges Orange flavour Dosage Adults : 1 lozenge every 3-6 hours, as needed. Do not exceed the dose of 8 lozenges in 24 hours. Paediatric population Children over 12 years of age: as for adults. Children under 12 years of age: do not administer to children under 12 years of age (see section 4.3). Special populations Elderly : the clinical data currently available are limited, therefore no recommendation on posology can be made. The elderly have an increased risk of serious consequences in case of adverse reactions (see section 4.4). Patients with hepatic impairment : no dosage reduction is necessary in patients with mild to moderate hepatic impairment. Flurbiprofen is contraindicated in patients with severe hepatic impairment (see section 4.3). Patients with renal impairment : no dosage reduction is necessary in patients with mild to moderate renal impairment. Flurbiprofen is contraindicated in patients with severe hepatic impairment (see section 4.3). Method of administration For oropharyngeal use. Dissolve slowly in the mouth. As with all lozenges, flurbiprofen lozenges should be moved around the mouth during administration to avoid local irritation. If mouth irritation occurs, treatment should be discontinued.CONSERVATION
Storage Benactiv Gola 0.25% spray for oral mucosa 15ml - How to store Benactiv Gola 0.25% spray for oral mucosa 15ml?
Benactiv Gola Sugar Free Lozenges Orange Flavour and Benactiv Gola Lemon and Honey Flavour Lozenges: store below 25°C.WARNINGS
Warnings Benactiv Gola 0.25% spray for oral mucosa 15ml - About Benactiv Gola 0.25% spray for oral mucosa 15ml it is important to know that:
At the recommended doses, when using the medicinal product in its various pharmaceutical forms, any swallowing does not cause any harm to the patient, as the dose of flurbiprofen is much lower than that commonly used in systemic treatments. Elderly: elderly patients have an increased frequency of adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation, which may be fatal. Respiratory disorders Cases of bronchospasm have been reported with flurbiprofen in patients with a history of bronchial asthma or allergies. Flurbiprofen should be used with caution in these patients. Other NSAIDs It is advisable not to combine the medicinal product with other NSAIDs (see section 4.5). Systemic lupus erythematosus (SLE) and mixed connective tissue disease Patients with systemic lupus erythematosus and mixed connective tissue disease may be at increased risk of aseptic meningitis (see section 4.8), however this effect is not usually seen with products intended for limited and short-term use such as flurbiprofen. Cardiac, hepatic and renal impairment The medicinal product should be used with caution in patients with cardiac, renal or hepatic insufficiency. NSAIDs have been reported to cause various forms of nephrotoxicity, including interstitial nephritis, nephrotic syndrome and renal failure. Administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and precipitate renal failure. Patients at greatest risk of developing this reaction are those with impaired renal function, cardiac impairment, hepatic dysfunction, those on diuretic therapy and the elderly; however, this effect is not usually seen with products intended for limited and short-term use such as flurbiprofen. Cardiovascular and cerebrovascular effects Caution is advised before initiating treatment in patients with a history of hypertension and/or heart failure (discuss with your doctor or pharmacist), as fluid retention, hypertension and oedema have been reported in association with NSAID treatment. Clinical trial and epidemiological data suggest that use of some NSAIDs, particularly at high doses and in long-term treatment, may be associated with a small increased risk of arterial thrombotic events such as myocardial infarction or stroke. There are insufficient data to exclude a similar risk for flurbiprofen. Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease and/or cerebrovascular disease should only be treated with flurbiprofen after careful consideration. Similar consideration should be made before initiating long-term treatment in patients with risk factors for cardiovascular disease (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking). Central nervous system effects Analgesic-induced headache. Headache may occur with prolonged or inappropriate use of analgesics and should not be treated by increasing the dose of the medicinal product. Gastrointestinal effects Flurbiprofen should be administered with caution to patients with a history of peptic ulcer and other gastrointestinal disease as these conditions may be exacerbated. The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing flurbiprofen dosage in patients with a history of ulcer, particularly if complicated with haemorrhage and perforation and in the elderly. These patients should start treatment on the lowest available dose. Gastrointestinal bleeding, ulceration or perforation have been reported with all NSAIDs at any time during treatment. These adverse reactions can be fatal and may occur with or without warning symptoms or in the presence of a previous history of serious gastrointestinal reactions. Patients with a history of gastrointestinal disease, particularly when elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding) in the early stages of treatment. Undesirable effects may be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms (see section 4.2). Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors or anti-platelet agents such as acetylsalicylic acid (see section 4.5). When gastrointestinal bleeding or ulceration occurs in patients receiving flurbiprofen, the treatment should be discontinued. Dermatological effects Use of the medicinal product, especially if prolonged, may give rise to sensitisation or local irritation phenomena. In such cases, treatment should be discontinued and a doctor should be consulted to institute appropriate therapy if necessary. Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs (see section 4.8). Flurbiprofen should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity. Infections Since isolated cases of exacerbation of infection-related inflammation (e.g. development of necrotising fasciitis) have been described in temporal association with systemic use of NSAIDs, patients are advised to seek immediate medical attention if signs of bacterial infection appear or worsen during flurbiprofen therapy. The possibility of initiating antibiotic therapy should be considered. If mouth irritation develops, treatment should be discontinued. BENACTIV GOLA Mouthwash and BENACTIV GOLA Spray contain para-hydroxy-benzoates which may cause allergic reactions (possibly delayed). BENACTIV GOLA Mouthwash and BENACTIV GOLA Spray contain 40-polyoxyethylened hydrogenated castor oil which may cause localised skin reactions. BENACTIV GOLA Mouthwash and BENACTIV GOLA Spray contain a flavouring which in turn contains d-limonene. D-limonene may cause allergic reactions. BENACTIV GOLA Mouthwash and BENACTIV GOLA Spray contain less than 1 mmol (23 mg) sodium per 10 ml dose, i.e. essentially “sodium-free”. BENACTIV GOLA Lemon and Honey flavoured pastilles contain liquid glucose, liquid sucrose and honey (invert sugar). Patients with rare problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. To be taken into account in people with diabetes mellitus: this medicine contains 1.07 g glucose and 1.41 g sucrose per lozenge. BENACTIV GOLA Lemon and Honey flavour lozenges contain sulphites. It can rarely cause serious hypersensitivity reactions and bronchospasm. BENACTIV GOLA Lemon and Honey flavour lozenges contain only a very small amount of gluten (from wheat starch). This medicine is considered “gluten-free” and is very unlikely to cause problems for a patient with coeliac disease. One lozenge contains no more than 21.38 micrograms of gluten. If a patient is allergic to wheat (a condition other than coeliac disease) they should not take this medicine. BENACTIV GOLA Lemon and Honey flavour lozenges contain a flavouring which in turn contains citral, citronellol, d-limonene, farnesol, geraniol and linalool. Citral, citronellol, d-limonene, farnesol, geraniol and linalool can cause allergic reactions. BENACTIV THROAT Lemon and Honey flavoured lozenges contain a flavouring containing butylated hydroxyanisole which can cause localised skin reactions (e.g. contact dermatitis) or irritation to the eyes and mucous membranes. BENACTIV THROAT Sugar-Free Lozenges Orange flavoured are instead indicated for those patients who need to control their sugar and calorie intake. BENACTIV THROAT Sugar-Free Lozenges Orange flavoured contain a flavouring containing citral, citronellol, d-limonene, geraniol and linalool. Citral, citronellol, d-limonene, geraniol and linalool can cause allergic reactions. BENACTIV THROAT Sugar-Free Lozenges Orange flavoured contains liquid maltitol and isomalt. Patients with rare hereditary problems of fructose intolerance should not take this medicine. It may have a mild laxative effect. The caloric value of maltitol and isomalt is 2.3 kcal/g. Do not use for prolonged treatments beyond 7 days. If no appreciable results are noted after 3 days of treatment, the cause may be a different pathological condition. In these cases, it is advisable to consult your doctor.INTERACTIONS
Interactions Benactiv Gola 0.25% spray for oral mucosa 15ml - Which medicines or foods can modify the effect of Benactiv Gola 0.25% spray for oral mucosa 15ml?
Caution should be exercised in patients treated with any of the following medicinal products, as interactions have been reported in some patients. Inform your doctor if you are taking other medicinal products. Flurbiprofen should be avoided in combination with: - Aspirin: unless low dose aspirin (not exceeding 100 mg/day or local prophylactic doses for cardiovascular protection) has been recommended by your doctor; as with other NSAID-containing medicinal products, concomitant administration of flurbiprofen and aspirin is generally not recommended due to the potential for increased adverse effects (see section 4.4). - Cox-2 inhibitors and other NSAIDs: concomitant use of other NSAIDs, including cyclooxygenase-2 selective inhibitors, should be avoided due to the potential for additive effects and an increased risk of adverse reactions (see section 4.4). Flurbiprofen should be used with caution in association with: - Anticoagulants: NSAIDs may enhance the effects of anticoagulants such as warfarin (see section 4.4) - Antiplatelet agents: increased risk of gastrointestinal bleeding - Selective serotonin reuptake inhibitors (SSRIs): increased risk of gastrointestinal bleeding - Antihypertensives (diuretics, ACE inhibitors and angiotensin II antagonists): NSAIDs may reduce the effect of diuretics. Other antihypertensive drugs may potentiate nephrotoxicity caused by inhibition of cyclooxygenase, especially in patients with impaired renal function (these patients should be adequately hydrated) - Alcohol: may increase the risk of adverse reactions, especially bleeding in the gastrointestinal tract - Cardiac glycosides: NSAIDs may exacerbate cardiac failure, reduce GFR (glomerular filtration rate) and increase plasma glycoside levels - Ciclosporin: increased risk of nephrotoxicity - Corticosteroids: increased risk of gastrointestinal ulceration or haemorrhage with NSAIDs (see section 4.4) - Lithium: there is evidence of a possible increase in lithium plasma levels - Methotrexate: there may be an increase in methotrexate plasma levels - Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration, as NSAIDs may reduce the effect of mifepristone. mifepristone - Quinolone antibiotics: Animal data indicate that NSAIDs may increase the risk of convulsions associated with quinolone antibiotics. Patients taking NSAIDs and quinolones may be at increased risk of developing convulsions - Tacrolimus: Possible increased risk of nephrotoxicity when NSAIDs are given with tacrolimus - Zidovudine: Increased risk of haematological toxicity when NSAIDs are given with zidovudine.SIDE EFFECTS
Like all medicines, Benactiv Gola 0.25% spray for oral mucosa 15ml can cause side effects - What are the side effects of Benactiv Gola 0.25% spray for oral mucosa 15ml?
Hypersensitivity reactions to NSAIDs have been reported and may consist of: (a) non-specific allergic reactions and anaphylaxis (b) respiratory tract reactivity, e.g. asthma, aggravated asthma, bronchospasm, dyspnoea (c) various skin disorders, including e.g. rashes of various types, pruritus, urticaria, purpura, angioedema and, more rarely, exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme). The most commonly observed adverse reactions are gastrointestinal in nature. Local use of the medicinal product, especially if prolonged, may give rise to sensitization or local irritation phenomena. Dissolution of the medicinal product in the oral cavity in tablet form may be accompanied by sensations of heat or tingling in the oropharynx. In such cases, treatment should be discontinued and, if necessary, appropriate therapy instituted. The following undesirable effects have been reported, particularly after administration of systemic formulations. These refer to those observed with the use of flurbiprofen used short-term and at doses compatible with the classification of over-the-counter medicines. In case of treatment of chronic conditions and over long periods of time, additional undesirable effects may occur. The undesirable effects associated with the use of flurbiprofen are divided below according to the system organ class and frequency. The frequency is defined as: very common (≥ 1/10), common (≥1/100, <1/10), uncommon (≥1/1,000, <1/100), rare (≥1/10,000, <1/1,000), very rare (<1/10,000) and not known (frequency cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.| Classification by systems and organs | Frequency | Adverse reactions |
| Pathologies of the haemolymphopoietic system | Not known | Anemia, thrombocytopenia, aplastic anemia and agranulocytosis |
| Pathologies of the nervous system | Common | Dizziness, headache, paresthesia |
| Uncommon | Drowsiness | |
| Not known | Cerebrovascular accident, optic neuritis, migraine, confusional states, vertigo | |
| Immune system disorders | Rare | Anaphylactic reaction |
| Not known | Angioedema, hypersensitivity | |
| Eye pathologies | Not known | Vision impairment |
| Ear and labyrinth pathologies | Not known | Tinnitus |
| Heart disease | Not known | Heart failure, edema |
| Vascular pathologies | Not known | Hypertension |
| Respiratory, thoracic and mediastinal pathologies | Common | Throat irritation |
| Uncommon | Asthma, bronchospasm and dyspnea, oropharyngeal vesicular eruption, oropharyngeal hypoesthesia | |
| Gastrointestinal disorders | Common | Diarrhoea, mouth ulceration, nausea, oral pain, oral paraesthesia, oropharyngeal pain, oral discomfort (sensation of heat or burning, tingling of the mouth) |
| Uncommon | Abdominal distension, abdominal pain, constipation, dry mouth, dyspepsia, flatulence, glossodynia, dysgeusia, oral dysesthesia, vomiting | |
| Not known | Melaena, haematemesis, gastrointestinal haemorrhage, colitis, exacerbation of Crohn's disease, gastritis, peptic ulcer, gastric perforation, ulcer haemorrhage | |
| Skin and subcutaneous tissue disorders | Uncommon | Skin rash, itching |
| Not known | Urticaria, purpura, bullous dermatitis (including Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme) | |
| Kidney and urinary disorders | Not known | Toxic nephropathy, tubulointerstitial nephritis and nephrotic syndrome, renal failure (as with other NSAIDs) |
| Systemic disorders and conditions related to the administration site | Uncommon | Pyrexia, pain |
| Not known | Discomfort, tiredness | |
| Hepatobiliary pathologies | Not known | Hepatitis |
| Psychiatric disorders | Uncommon | Insomnia |
| Not known | Depression, hallucination |
OVERDOSE
Overdose Benactiv Gola 0.25% spray for oral mucosa 15ml - What are the risks of Benactiv Gola 0.25% spray for oral mucosa 15ml in case of overdose?
In view of the low active ingredient content and its local use, overdose is unlikely to occur. Symptoms The majority of patients who ingest clinically important quantities of NSAIDs develop nausea, vomiting, gastrointestinal irritation, epigastric pain, or more rarely diarrhoea. Tinnitus, headache and gastrointestinal bleeding are also possible. In more severe cases of NSAID intoxication, central nervous system toxicity is observed, manifested by drowsiness, occasionally excitability, blurred vision and disorientation or coma. Occasionally patients develop convulsions. In severe NSAID intoxication, metabolic acidosis may occur and the prothrombin time/INR may be prolonged, probably due to interference with the action of circulating coagulation factors. Acute renal failure and liver damage may occur. An exacerbation of asthma is possible in asthmatic subjects. Treatment Treatment should be symptomatic and supportive and should include maintaining a patent airway and monitoring cardiac function and vital signs until stable. Oral administration of activated charcoal and, if necessary, correction of serum electrolytes should be considered if the patient presents within 1 hour of ingestion of a potentially toxic amount. Seizures should be treated with intravenous diazepam or lorazepam if frequent or prolonged. Bronchodilators for asthma should be administered. There is no specific antidote for flurbiprofen.PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Benactiv Gola 0.25% spray for oral mucosa 15ml
Pregnancy Flurbiprofen should not be administered during the first and second trimester of pregnancy unless clearly necessary. The use of flurbiprofen during the third trimester of pregnancy is contraindicated. Breastfeeding In a limited number of studies, flurbiprofen appears in breast milk at very low concentrations and is unlikely to have any adverse effects on the breast-fed infant. However, administration of flurbiprofen is not recommended in nursing mothers. Fertility There is evidence that cyclooxygenase/prostaglandin synthesis inhibitors may cause impairment of female fertility by an effect on ovulation. This is reversible upon discontinuation of treatment.DRIVING AND USE OF MACHINERY
Taking Benactiv Gola 0.25% spray for oral mucosa 15ml before driving or using machines - Does Benactiv Gola 0.25% spray for oral mucosa 15ml affect driving and using machines?
It does not interfere with the ability to drive vehicles or use machinery.