CASEN RECORDATI SL
Fleet Enema 21.4g/9.4g Rectal Solution Ready to Use 4 Bottles 133ml
Fleet Enema 21.4g/9.4g Rectal Solution Ready to Use 4 Bottles 133ml
Regular price
€17,70
Regular price
€17,70
Sale price
€17,70
Unit price
per
Tax included.
Shipping calculated at checkout.

Pickup available at Farmacia Tili
Usually ready in 24 hours
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
EAN
EAN
029319023
MINSAN
MINSAN
029319023
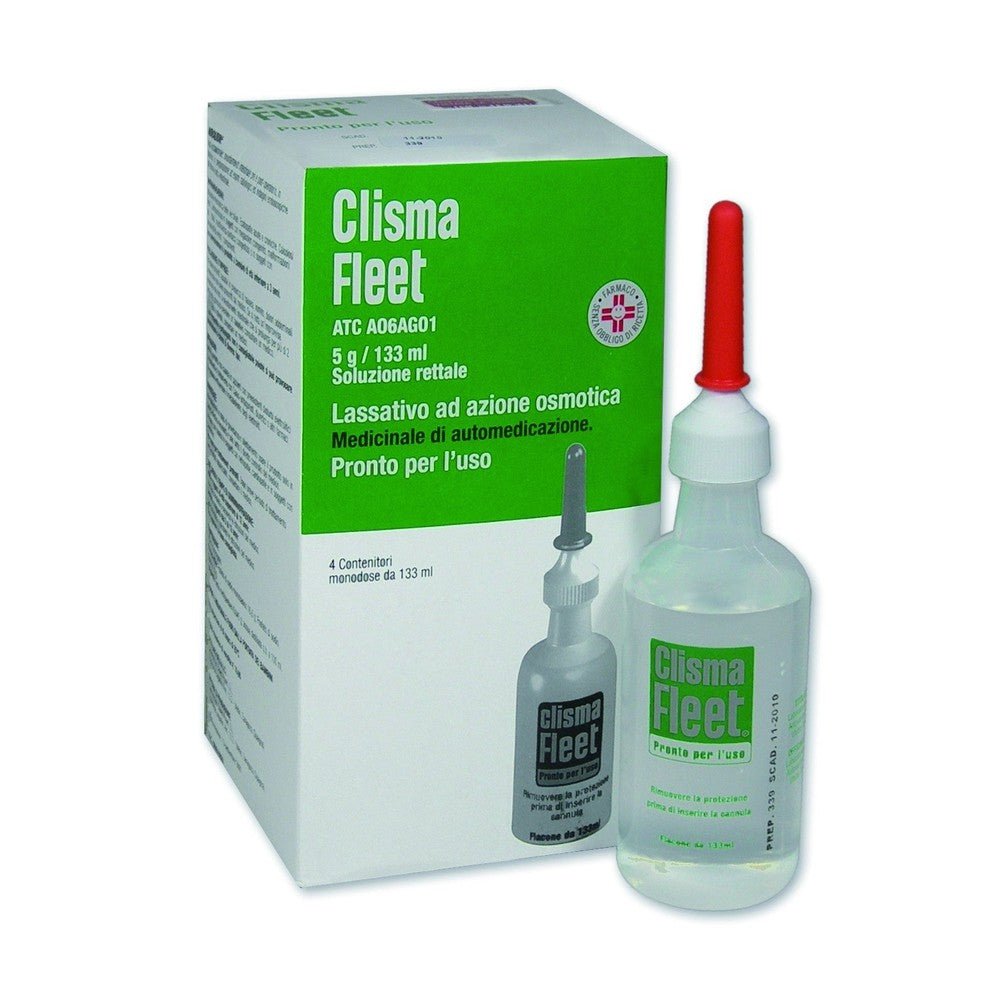
Fleet Enema 21.4g/9.4g ready-to-use rectal solution 4 bottles 133ml is a medical device designed for the short-term treatment of occasional constipation in adults and children over 3 years of age. This product is particularly indicated for pre- and post-operative intestinal emptying , in obstetrics, and in preparation for radioscopic examinations and endoscopic investigations of the lower intestinal tract. Each dose of 118 ml contains the equivalent of 21.4 g of sodium dihydrogen phosphate dihydrate and 9.4 g of disodium hydrogen phosphate dodecahydrate , ensuring an effective laxative action. The formulation is enriched with benzalkonium chloride , an excipient known for its preservative properties. The product is packaged in practical 133 ml bottles, ready for use, which ensure simple and quick application.
ACTIVE INGREDIENTS
Active ingredients contained in Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - What is the active ingredient in Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml?
Each 118 ml dose contains the equivalent of 21.4 g (18.1% w/v) sodium dihydrogen phosphate dihydrate and 9.4 g (8.0% w/v) disodium hydrogen phosphate dodecahydrate. Contains 4.4 g sodium per 118 ml delivered dose. Excipient with known effect: This medicinal product contains 0.0006 g/ml benzalkonium chloride. For the full list of excipients, see section 6.1.EXCIPIENTS
Composition of Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - What does Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml contain?
Benzalkonium chloride Disodium edetate Distilled water qsDIRECTIONS
Therapeutic indications Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - Why is Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml used? What is it used for?
Short-term treatment of occasional constipation in adults and children over 3 years of age; pre- and post-operative bowel emptying, in obstetrics, in preparation for radioscopic examinations and endoscopic investigations of the lower intestinal tract.CONTRAINDICATIONS SIDE EFFECTS
Contraindications Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - When should Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml not be used?
Fleet enema is contraindicated in patients with: • Hypersensitivity to the active substances or to any of the excipients listed in section 6.1. • Conditions causing increased absorption capacity, decreased elimination capacity or decreased gastric motility, for example: o suspected intestinal obstruction o paralytic ileus o anorectal stenosis o perforated anus o congenital or acquired megacolon o Hirschsprung's disease. • Undiagnosed gastrointestinal disorders, for example: o symptoms suggestive of appendicitis, intestinal perforation or active inflammatory bowel disease o undiagnosed rectal bleeding. • Congestive heart failure. • Dehydration. • Children under 3 years of age. • Clinically significant renal insufficiency. Other preparations containing sodium phosphate, including oral solutions or sodium phosphate tablets, should not be administered concomitantly (see section 4.5).DOSAGE
Quantity and method of taking Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - How do you take Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml?
Dosage Adults and children over 12 years of age: 1 bottle (118 ml delivered dose) no more than once a day or as directed by your doctor (see section 4.4). Children aged 3 to 12 years: Halved dose no more than once a day or as directed by your doctor (see sections 4.4 and 4.9). Fleet Enema is contraindicated in children under 3 years of age (see section 4.3). In occasional constipation, laxatives should be used as infrequently as possible and for no more than seven days. Use for longer periods of time requires a doctor's prescription after adequate assessment of the case. Renal insufficiency: Do not administer to patients with clinically significant impairment of renal function (see section 4.3). The product should be used with caution in patients with impaired renal function, when the clinical benefit is expected to outweigh the risk of hyperphosphatemia (see section 4.4). Method of administration For rectal use only: Lie on your left side with both knees bent, arms at rest. Remove the orange protective cap. Using constant pressure, gently insert the Comfortip enema bottle into the anus with the cannula pointing toward the navel. Squeeze the bottle until almost all the liquid has come out. Discontinue use if resistance is encountered. Forcing the enema may cause injury. 2 to 5 minutes is sufficient to achieve the desired effect. For occasional constipation, rectal enemas are used to provide relief and only in the short term.CONSERVATION
Storage Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - How do you store Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml?
Do not store above 25°C.WARNINGS
Warnings Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - It is important to know that about Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml:
Do not use Fleet Enema if nausea, vomiting or pain occurs. Patients should be advised to expect loose stools and encouraged to drink fluids to help prevent dehydration, particularly patients with conditions that may predispose them to dehydration or those taking medications that may decrease the glomerular filtration rate, such as diuretics, angiotensin converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs) or non-steroidal anti-inflammatory drugs (NSAIDs). As Fleet Enema contains sodium phosphates, there is a risk of elevated serum sodium and phosphate levels and decreased calcium and potassium levels and consequently hypernatraemia, hyperphosphatemia, hypocalcaemia and hypokalaemia which may occur with clinical signs such as tetany and renal failure. Fluid and electrolyte changes are of particular concern in children with megacolon or any other condition where retention of the enema solution occurs, and in patients with co-morbidities. This is why Fleet Enema should be used with caution in: • elderly or debilitated patients and in patients with uncontrolled arterial hypertension, ascites, heart disease, changes in the rectal mucosa (ulcers, fissures); • subjects with a colostomy, patients taking diuretics or other drugs that can alter fluid and electrolyte levels; • subjects taking drugs known to prolong the QT interval (e.g. amiodarone, arsenic trioxide, astemizole, azithromycin, erythromycin, clarithromycin, chlorpromazine, cisapride, citalopram, domperidone, terfenadine, procainamide), or with known fluid and electrolyte imbalance, such as hypocalcemia, hypokalemia, hyperphosphatemia, hypernatremia. Also use with caution in patients taking drugs known to affect perfusion, renal function, or hydration status. Where fluid and electrolyte disturbances and risk of hyperphosphataemia are suspected, fluid and electrolyte levels should be monitored before and after administration of Fleet Enema. The product should be used with caution in patients with impaired renal function, when the clinical benefit is expected to outweigh the risk of hyperphosphataemia. Repeated and prolonged use of Fleet Enema is not recommended as it may cause habituation. Administration of more than one enema in a 24-hour period may be harmful. Fleet Enema should not be used for more than one week. Fleet Enema should be administered according to the instructions for use (see section 4.2). Patients should be advised to discontinue administration if resistance is encountered, as forceful administration of the enema may cause injury. Rectal bleeding after use of Fleet Enema may indicate a serious condition. In this case, administration should be stopped immediately. In general, evacuation occurs approximately 5 minutes after administration of Fleet Enema, therefore, retention times longer than 5 minutes are not recommended. If evacuation does not occur after using Fleet Enema or if the retention time lasts more than 10 minutes, serious adverse effects may occur. No other administration should be used and the patient's condition should be evaluated in order to identify any hydroelectrolytic alterations and to minimize the risk of severe hyperphosphataemia (see sections 4.8 and 4.9). Important information about some of the excipients Due to the presence of benzalkonium chloride, the product is irritant and may cause local skin reactions.INTERACTIONS
Interactions Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - Which medicines or foods can modify the effect of Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml?
Use with caution in patients receiving calcium antagonists, diuretics, lithium or other medicinal products that may affect fluid and electrolyte levels as hyperphosphataemia, hypocalcaemia, hypokalaemia, acidosis and hypernatraemic dehydration may occur (see section 4.4). Other preparations containing sodium phosphates including oral solutions or sodium phosphate tablets should not be administered concomitantly (see section 4.3). Since hypernatraemia is associated with low lithium levels, concomitant use of Fleet Enema and lithium may cause a reduction in serum lithium levels with a decrease in efficacy.SIDE EFFECTS
Like all medicines, Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml can cause side effects - What are the side effects of Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml?
Fleet Enema is well tolerated when used as directed. However, adverse events associated with the use of Fleet Enema have been frequently reported. In some cases, adverse events may occur, especially if the enema is used inappropriately. Within each frequency grouping, undesirable effects are presented in decreasing order of seriousness. Undesirable effects are listed below, by MedDRA system organ class and using the following frequency: very common (≥ 1/10), common (≥ 1/100, <1/10), uncommon (≥ 1/1,000, <1/100), rare (≥ 1/10,000, <1/1,000), very rare (<1/10,000), not known (frequency cannot be estimated from the available data): Immune system disorders : Very rare: hypersensitivity e.g. urticaria. Skin and subcutaneous tissue disorders : Very rare: vesicles, pruritus, burning. Metabolism and nutrition disorders : Very rare: dehydration, hyperphosphatemia, hypocalcemia, hypokalaemia, hypernatremia, metabolic acidosis. Gastrointestinal disorders : Very rare: nausea, vomiting, abdominal pain, abdominal distension, diarrhoea, gastrointestinal pain, anal discomfort and proctalgia. Systemic disorders and administration site conditions : Very rare: rectal irritation, pain, burning, chills. Reporting of suspected adverse reactions Reporting suspected adverse reactions that occur after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system at www.agenziafarmaco.gov.it/it/responsabili.OVERDOSE
Overdose Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml - What are the risks of Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml in case of overdose?
Fatalities have been reported following administration of excessive doses, after retention, in pediatric patients, or in obstructed patients. Hyperphosphatemia, hypocalcemia, hypernatremia, hyperphosphatemia, hypernatremic dehydration, hypokalemia, hypovolemia, acidosis, and tetany may occur following overdosage or retention. Recovery from toxic effects can usually be achieved by rehydration. Treatment of electrolyte imbalances may require immediate medical intervention by administration of adequate electrolytes and fluid therapy.PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml
Since there are no relevant data available to evaluate the potential for fetal malformation or other fetotoxic effects when administered during pregnancy, Fleet Enema should be used under the direct supervision of a physician only at the time of delivery or postpartum. Since sodium phosphate may pass into breast milk, it is recommended that breast milk be expressed and discarded for at least 24 hours after administering Fleet Enema.DRIVING AND USE OF MACHINERY
Taking Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml before driving or using machines - Does Fleet Enema 21.4g/9.4g ready-to-use rectal solution 133ml affect driving or using machines?
Fleet Enema does not alter the ability to drive or use machines, but it is necessary to remain near the toilet after using this medicine.