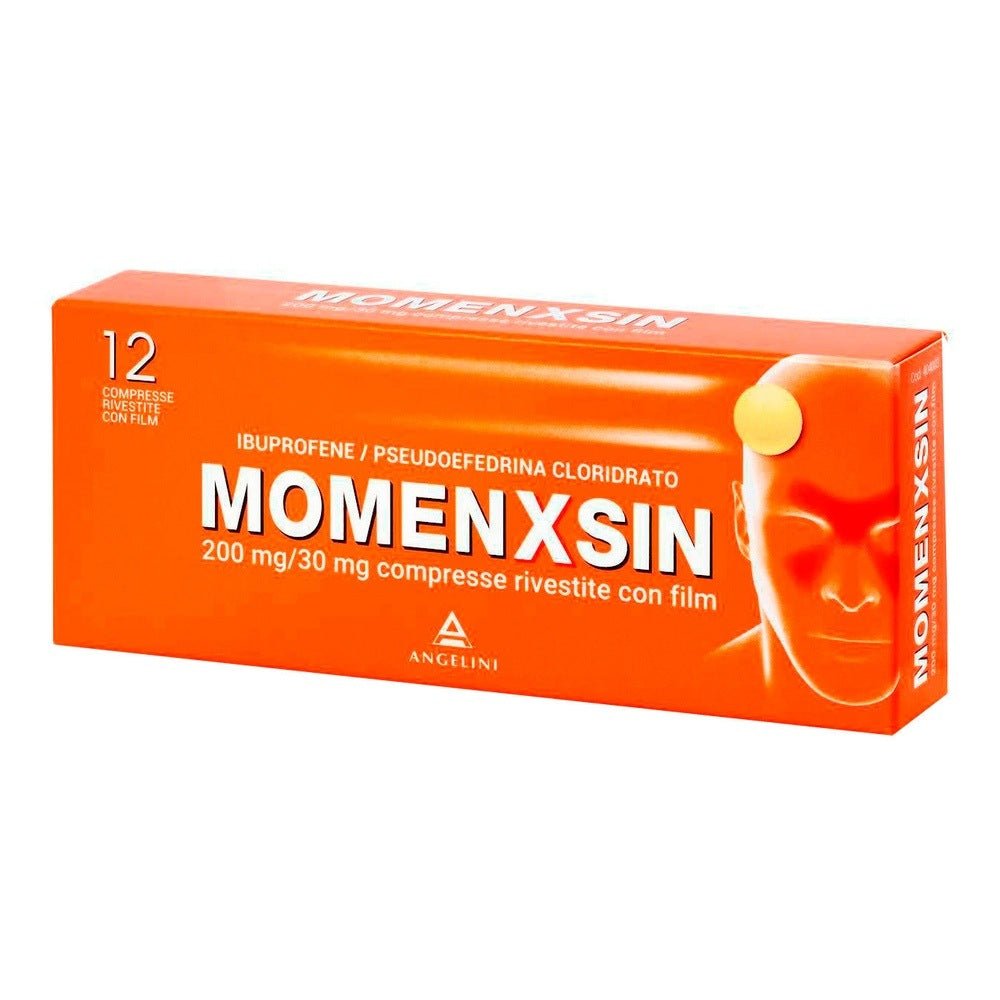
ANGELINI PHARMA ITALIA SpA
Momenxsin 200mg/30mg 12 coated tablets
Momenxsin 200mg/30mg 12 coated tablets

Pickup available at Farmacia Tili
Usually ready in 24 hours
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
EAN
EAN
043682020
MINSAN
MINSAN
043682020
Momenxsin 200mg/30mg 12 coated tablets is a combination medicine that combines the properties of ibuprofen and pseudoephedrine hydrochloride . Each coated tablet contains 200 mg of ibuprofen , a well-known nonsteroidal anti-inflammatory drug (NSAID) that helps reduce pain and inflammation, and 30 mg of pseudoephedrine hydrochloride , a decongestant that relieves nasal congestion. This product is indicated for the symptomatic treatment of nasal congestion associated with acute rhinosinusitis of suspected viral origin, accompanied by headache and/or fever . Momenxsin is intended for use in adults and adolescents aged 15 years and older. The tablets are coated to facilitate swallowing and improve gastrointestinal tolerability.
ACTIVE INGREDIENTS
Active ingredients contained in Momenxsin 200mg/30mg 12 coated tablets - What is the active ingredient of Momenxsin 200mg/30mg 12 coated tablets?
Each film-coated tablet contains 200 mg ibuprofen and 30 mg pseudoephedrine hydrochloride. Excipient with known effect: sodium. For the full list of excipients, see section 6.1.
EXCIPIENTS
Composition of Momenxsin 200mg/30mg 12 coated tablets - What does Momenxsin 200mg/30mg 12 coated tablets contain?
Tablet core: Microcrystalline cellulose; Calcium hydrogen phosphate anhydrous; Croscarmellose sodium ; Maize starch; Colloidal anhydrous silica; Magnesium stearate. Tablet coating: Hypromellose; Macrogol 400; Talc; Titanium dioxide (E171); Yellow iron oxide (E172).
DIRECTIONS
Therapeutic indications Momenxsin 200mg/30mg 12 coated tablets - Why is Momenxsin 200mg/30mg 12 coated tablets used? What is it used for?
Symptomatic treatment of nasal congestion associated with acute rhinosinusitis of suspected viral origin with headache and/or fever. Momenxsin is indicated in adults and adolescents aged 15 years and older.
CONTRAINDICATIONS SIDE EFFECTS
Contraindications Momenxsin 200mg/30mg 12 coated tablets - When should Momenxsin 200mg/30mg 12 coated tablets not be used?
• Hypersensitivity to ibuprofen, pseudoephedrine hydrochloride or to any of the excipients listed in section 6.1; • Patients under 15 years of age; • Women in the third trimester of pregnancy (see section 4.6); • Breast-feeding women (see section 4.6); • Patients with a history of hypersensitivity reactions (e.g. bronchospasm, asthma, rhinitis, angioedema or urticaria) associated with acetylsalicylic acid or other nonsteroidal anti-inflammatory drugs (NSAIDs); • History of gastrointestinal bleeding or perforation associated with previous NSAID therapy; • Current or history of recurrent peptic ulcer/haemorrhage (at least two distinct episodes of proven ulceration or bleeding); • Cerebrovascular or other bleeding; • Haematopoietic abnormalities of unknown origin; • Severe hepatic insufficiency; • Severe renal insufficiency; • Severe cardiac insufficiency; • Severe cardiovascular disorders, coronary heart disease (heart disease, hypertension, angina pectoris), tachycardia, hyperthyroidism, diabetes, pheochromocytoma; • History of stroke or presence of risk factors for stroke (due to the α-–sympathomimetic activity of pseudoephedrine hydrochloride); • Risk of narrow-angle glaucoma; • Risk of urinary retention related to urethroprostatic disorders; • History of myocardial infarction; • History of seizures; • Systemic lupus erythematosus; • Concomitant use of other vasoconstrictors such as nasal decongestants, administered orally or nasally (e.g. phenylpropanolamine, phenylephrine and ephedrine), and methylphenidate (see section 4.5); • Concomitant use of non-selective monoamine oxidase inhibitors (MAOIs) (iproniazid) (see section 4.5) or use of monoamine oxidase inhibitors within the last two weeks.
DOSAGE
Quantity and method of taking Momenxsin 200mg/30mg 12 coated tablets - How to take Momenxsin 200mg/30mg 12 coated tablets?
Dosage. Adults and adolescents aged 15 years and over : 1 tablet (equivalent to 200 mg ibuprofen and 30 mg pseudoephedrine hydrochloride) every 6 hours if necessary. In case of more intense symptoms, 2 tablets (equivalent to 400 mg ibuprofen and 60 mg pseudoephedrine hydrochloride) every 6 hours if necessary, up to a maximum total daily dose of 6 tablets (equivalent to 1200 mg ibuprofen and 180 mg pseudoephedrine hydrochloride). Do not exceed the maximum total daily dose of 6 tablets (equivalent to 1200 mg ibuprofen and 180 mg pseudoephedrine hydrochloride). For short-term use. If symptoms worsen, consult a doctor. The maximum duration of treatment is 4 days for adults and 3 days for adolescents aged 15 years and over. In cases where the symptoms consist predominantly of pain/fever or nasal congestion, the administration of a product containing a single active ingredient is preferable. Undesirable effects can be limited by using the lowest effective dose for the shortest time necessary to control the symptoms (see section 4.4). Paediatric population : Momenxsin is contraindicated in paediatric patients under 15 years of age (see section 4.3). Method of administration: For oral use. The tablets should be swallowed whole and not chewed, with a glass of water, preferably during meals.
CONSERVATION
Storage Momenxsin 200mg/30mg 12 coated tablets - How to store Momenxsin 200mg/30mg 12 coated tablets?
Do not store above 30°C.
WARNINGS
Warnings Momenxsin 200mg/30mg 12 coated tablets - About Momenxsin 200mg/30mg 12 coated tablets it is important to know that:
Concomitant use of Momenxsin and other NSAIDs, including selective cyclooxygenase (COX)-2 inhibitors, should be avoided. Undesirable effects may be minimised by using the lowest effective dose necessary to control symptoms for the shortest duration possible (see sections "Gastrointestinal effects" and "Cardiovascular and cerebrovascular effects" below). If symptoms persist beyond the maximum recommended duration of treatment with this medicinal product (4 days for adults and 3 days for adolescents), the measures to be implemented, in particular the possible usefulness of antibiotic treatment, should be reassessed. Acute rhinosinusitis of suspected viral origin is defined as a series of bilateral rhinological symptoms of moderate intensity, dominated by nasal congestion with severe or purulent rhinorrhoea, occurring in an epidemic context. The purulent aspect of rhinorrhoea is common and does not systematically correspond to bacterial superinfection. Sinus pain in the first few days of the disease is associated with congestion of the sinus mucosa (acute congestive rhinosinusitis) and resolves spontaneously in most cases. In case of acute bacterial sinusitis, antibiotic therapy is justified. Special warnings related to pseudoephedrine hydrochloride : • The dosage, the maximum recommended duration of treatment (4 days for adults and 3 days for adolescents) and contraindications must be strictly respected (see section 4.8); • Patients should be informed that treatment must be discontinued if hypertension, tachycardia, palpitations, cardiac arrhythmias, nausea or any neurological signs, such as the onset or worsening of headache, appear; • Ischemic colitis Some cases of ischemic colitis have been reported with pseudoephedrine. If sudden abdominal pain, rectal bleeding or other symptoms of ischemic colitis develop, pseudoephedrine should be discontinued and a doctor should be consulted. Serious skin reactions: Serious skin reactions such as acute generalized exanthematous pustulosis (AGEP) may occur with products containing pseudoephedrine. This acute pustular eruption may occur within the first 2 days of treatment, with fever and numerous, small, mostly non-follicular pustules arising from a widespread edematous erythema and located mainly on the skin folds, trunk and upper limbs. Patients should be carefully monitored. If signs and symptoms such as pyrexia, erythema or numerous small pustules are observed, administration of Momenxsin should be discontinued and appropriate measures taken if necessary. Before using this medicine, patients should consult their doctor in case of: • hypertension, heart disease, hyperthyroidism, psychosis or diabetes; • concomitant use of antimigraine drugs, in particular vasoconstrictors based on ergot alkaloids (due to the α-sympathomimetic activity of pseudoephedrine); • mixed connective tissue disease: increased risk of aseptic meningitis (see section 4.8); • neurological symptoms such as seizures, hallucinations, behavioral disturbances, agitation and insomnia have been described following the administration of systemic vasoconstrictors, especially during febrile episodes or in case of overdose. These symptoms have been reported more commonly in the pediatric population. Therefore, it is advisable to: • avoid the administration of Momenxsin in association with drugs that can lower the epileptogenic threshold, such as terpene derivatives, clobutinol, atropine-like substances and local anaesthetics, or in the presence of a history of seizures; • strictly adhere, in all cases, to the recommended dosage and inform patients about the risks of overdose if Momenxsin is taken concomitantly with other medicinal products containing vasoconstrictors. Patients with urethroprostatic disorders are more likely to develop symptoms such as dysuria and urinary retention. Elderly patients may be more sensitive to the effects on the central nervous system (CNS). Precautions for use relating to pseudoephedrine hydrochloride : • In patients who are to undergo scheduled surgery in which volatile halogenated anaesthetics are to be used, it is preferable to discontinue treatment with Momenxsin several days before the operation, in view of the risk of acute hypertension (see section 4.5); • Athletes should be informed that treatment with pseudoephedrine hydrochloride may result in positive anti-doping tests. Interference with serological tests: Pseudoephedrine may potentially reduce the uptake of iobenguane i-131 in neuroendocrine tumors, thus interfering with scintigraphy. Special warnings relating to ibuprofen : In patients suffering from bronchial asthma or allergic diseases or with a history of such diseases, bronchospasm may occur. In case of asthma, the product should not be taken without first consulting a doctor (see section 4.3). Patients with asthma associated with chronic rhinitis, chronic sinusitis and/or nasal polyposis are at increased risk of allergic reactions when taking acetylsalicylic acid and/or NSAIDs. Administration of Momenxsin may trigger an acute asthma attack, particularly in some patients allergic to acetylsalicylic acid or an NSAID (see section 4.3). Prolonged use of any type of painkiller for headache may cause worsening of the headache. If this situation is observed or suspected, medical advice should be sought and treatment discontinued. The diagnosis of medication-overuse headache (MOH) should be suspected in patients who experience frequent or daily headaches despite (or because of) the regular use of headache medications. Patients with blood clotting disorders should consult their doctor before using this medicine. Gastrointestinal effects: Gastrointestinal bleeding, ulceration or perforation, sometimes fatal, have been reported with all NSAIDs at any time during treatment, with or without warning symptoms or a previous history of GI events. The risk of gastrointestinal bleeding, ulceration or perforation, sometimes fatal, is increased with increasing NSAID doses, in patients with a history of ulcer, particularly if complicated with bleeding or perforation (see section 4.3), and in the elderly. These patients should start treatment on the lowest dose available. Combination therapy with gastroprotective agents (e.g. misoprostol or proton pump inhibitors) should be considered for these patients and for those taking concomitant low dose acetylsalicylic acid or other medicinal products likely to increase gastrointestinal risk (see below and section 4.5). Patients with a history of gastrointestinal toxicity, particularly when elderly, should report any unusual abdominal symptoms (particularly gastrointestinal bleeding), particularly in the initial stages of treatment. Particular caution is advised in patients receiving concomitant treatment with drugs which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors (SSRIs) or anti-platelet agents such as acetylsalicylic acid (see section 4.5). Treatment with Momenxsin should be discontinued immediately if gastrointestinal bleeding or ulceration occurs. NSAIDs should be administered with caution to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) as these conditions may be exacerbated (see section 4.8). In case of concomitant intake of alcohol, the use of NSAIDs may increase the undesirable effects related to the active substance, in particular those involving the gastrointestinal tract or the central nervous system. Cardiovascular and cerebrovascular effects: The following conditions are contraindicated due to the presence of pseudoephedrine hydrochloride (see section 4.3): severe cardiovascular disorders, coronary heart disease (heart disease, hypertension, angina pectoris), tachycardia, hyperthyroidism, diabetes, pheochromocytoma, history of stroke or presence of risk factors for stroke, history of myocardial infarction. Clinical trials suggest that use of ibuprofen, particularly at high doses (2400 mg/day), may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke). Overall, epidemiological studies do not suggest that low-dose ibuprofen (e.g. ≤ 1200 mg/day) is associated with an increased risk of arterial thrombotic events. Patients with uncontrolled hypertension, heart failure (NYHA II-III), established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with ibuprofen after careful consideration and high doses (2400 mg/day) should be avoided. Careful consideration should also be given before patients with risk factors for cardiovascular events (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking) undertake long-term treatment, particularly if high doses of ibuprofen (2400 mg/day) are required. Severe skin reactions: Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs (see section 4.8). Patients appear to be at highest risk for these reactions in the early stages of therapy: the onset of the reaction occurs in most cases within the first month of treatment. Severe skin reactions, such as acute generalized exanthematous pustulosis (AGEP), may occur with medicinal products containing ibuprofen and pseudoephedrine. This acute pustular eruption may occur within the first 2 days of treatment, with fever and numerous, small, mostly non-follicular pustules, arising from a widespread edematous erythema and localized mainly in the skin folds, trunk and upper limbs. Patients should be carefully monitored. If signs and symptoms such as pyrexia, erythema or numerous small pustules, as well as the appearance of a skin rash, mucosal lesions or any other sign of hypersensitivity are observed, the administration of Momenxsin should be discontinued and appropriate measures taken if necessary. Masking of symptoms of underlying infections: Momenxsin may mask the symptoms of infection, which may delay initiation of adequate treatment and therefore worsen the outcome of the infection. This has been observed in community-acquired bacterial pneumonia and bacterial complications of chickenpox. When Momenxsin is administered for the relief of infection-related fever or pain, monitoring for infection is advised. In non-hospital settings, the patient should seek medical attention if symptoms persist or worsen. Precautions for use related to ibuprofen: • Elderly: The pharmacokinetics of ibuprofen are not affected by age, therefore no dosage adjustment is necessary in the elderly. However, elderly patients should be carefully monitored, as they are more sensitive to adverse reactions related to NSAIDs, in particular gastrointestinal bleeding and perforation, which may be fatal; • Caution and special monitoring are required when ibuprofen is administered to patients with a history of gastrointestinal disease (such as peptic ulcer, hiatus hernia or gastrointestinal bleeding); • In the initial stages of treatment, careful monitoring of diuresis and renal function is necessary in patients with heart failure, in patients with chronic renal or hepatic impairment, in patients taking diuretics, in patients who are hypovolaemic due to major surgery and, in particular, in elderly patients. Dehydrated adolescents are at risk of renal damage; • If vision changes during treatment, the patient should undergo a complete ophthalmological examination. Important information about some of the excipients. Momenxsin contains: - Sodium: this medicine contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially 'sodium-free'.
INTERACTIONS
Interactions Momenxsin 200mg/30mg 12 coated tablets - Which medicines or foods can modify the effect of Momenxsin 200mg/30mg 12 coated tablets?
| Association of pseudoephedrine with: | Possible reaction |
| Non-selective MAOIs (iproniazid) | Paroxysmal hypertension and hyperthermia, which may be fatal. Due to the long duration of action of MAOIs, this interaction may occur up to 15 days after discontinuation of the MAOI. |
| Other sympathomimetics or indirectly acting vasoconstrictors administered orally or nasally, α-sympathomimetic drugs, phenylpropanolamine, phenylephrine, ephedrine, methylphenidate | Risk of vasoconstriction and/or hypertensive crisis. |
| Reversible inhibitors of monoamine oxidase A (RIMA), linezolid, dopaminergic ergot alkaloids, vasoconstrictor ergot alkaloids | Risk of vasoconstriction and/or hypertensive crisis. |
| Volatile halogenated anesthetics | Acute perioperative hypertension. In case of scheduled surgery, stop taking Momenxsin several days in advance. |
| Guanethidine, reserpine and methyldopa | The effect of pseudoephedrine may be attenuated. |
| Tricyclic antidepressants | The effect of pseudoephedrine may be attenuated or enhanced. |
| Digitalis, quinidine or tricyclic antidepressants | Increased frequency of arrhythmias. |
| Concomitant use of ibuprofen with: | Possible reaction |
| Other NSAIDs, including salicylates and selective COX-2 inhibitors | Concomitant administration of several NSAIDs may increase the risk of bleeding and gastrointestinal ulcers due to a synergistic effect. Therefore, concomitant use of ibuprofen with other NSAIDs should be avoided (see section 4.4). |
| Digoxin | Concomitant use of Momenxsin with digoxin preparations may increase the serum levels of the latter. With correct use (maximum 4 days), a control of serum digoxin levels is generally not necessary. |
| Corticosteroids | Corticosteroids may increase the risk of adverse reactions, particularly affecting the gastrointestinal tract (gastrointestinal bleeding or ulceration) (see section 4.3). |
| Anti-platelet agents | Increased risk of gastrointestinal bleeding (see section 4.4). |
| Acetylsalicylic acid | Concomitant administration of ibuprofen and acetylsalicylic acid is generally not recommended due to the potential for increased adverse effects. Experimental data suggest that ibuprofen may cause competitive inhibition of the effect of low-dose acetylsalicylic acid on platelet aggregation when the two medicinal products are administered concomitantly. Although there are doubts as to the applicability of these data to clinical situations, the possibility that long-term regular use of ibuprofen may reduce the cardioprotective effect of low-dose acetylsalicylic acid cannot be excluded. No clinically relevant effect is considered likely following occasional use of ibuprofen (see section 5.1). |
| Anticoagulants (e.g. warfarin, ticlopidine, clopidogrel, Tirofiban, eptifibatide, abciximab, iloprost) | NSAIDs such as ibuprofen may enhance the effects of anticoagulants (see section 4.4). |
| Phenytoin | Concomitant use of Momenxsin with phenytoin preparations may increase the serum levels of the latter. With correct use (maximum 4 days), a control of phenytoin serum levels is generally not necessary. |
| Selective serotonin reuptake inhibitors (SSRIs) | Increased risk of gastrointestinal bleeding (see section 4.4). |
| Lithium | Concomitant use of Momenxsin with lithium preparations may increase the serum levels of the latter. With correct use (maximum 4 days), a control of serum lithium levels is generally not necessary. |
| Probenecid and sulfinpyrazone | Medicines containing probenecid or sulfinpyrazone may delay the excretion of ibuprofen. |
| Diuretics, ACE inhibitors, beta-blockers and angiotensin II antagonists | NSAIDs may reduce the effect of diuretics and other antihypertensive drugs. In some patients with compromised renal function (e.g. dehydrated patients or elderly patients with compromised renal function), the concomitant administration of ACE inhibitors, beta-blockers or angiotensin II antagonists and agents that inhibit cyclo-oxygenase may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible. Therefore, this combination should be administered with caution, especially in the elderly. Patients should be adequately hydrated and consideration should be given to monitoring renal function after initiation of concomitant therapy and periodically thereafter. |
| Potassium-sparing diuretics | Concomitant administration of Momenxsin and potassium-sparing diuretics may cause hyperkalemia (monitoring of serum potassium levels is recommended). |
| Methotrexate | Administration of Momenxsin within 24 hours before or after taking methotrexate may increase its concentrations and toxic effects. |
| Cyclosporine | The risk of renal damage induced by ciclosporin is increased by the concomitant use of some nonsteroidal anti-inflammatory drugs. This effect cannot be excluded even in the case of concomitant intake of ciclosporin and ibuprofen. |
| Tacrolimus | The risk of nephrotoxicity increases if the two drugs are administered concomitantly. |
| Zidovudine | There is evidence of an increased risk of haemarthrosis and haematoma in HIV (+) haemophiliac patients receiving concomitant treatment with zidovudine and ibuprofen. |
| Sulfonylureas | Clinical studies have demonstrated the existence of interactions between nonsteroidal anti-inflammatory drugs and antidiabetic drugs (sulfonylureas). Although no interactions between ibuprofen and sulfonylureas have been described so far, when these two drugs are taken at the same time, it is advisable, as a precaution, to monitor blood sugar levels. |
| Quinolone antibiotics | Animal studies indicate that NSAIDs may increase the risk of seizures associated with quinolone antibiotics. Patients taking NSAIDs and quinolones may be at increased risk of seizures. |
| Heparin; gingko biloba | Increased risk of bleeding. |
SIDE EFFECTS
Like all medicines, Momenxsin 200mg/30mg 12 coated tablets can cause side effects - What are the side effects of Momenxsin 200mg/30mg 12 coated tablets?
The most commonly observed adverse reactions related to ibuprofen are gastrointestinal in nature. Peptic ulcers, perforation or gastrointestinal bleeding, even fatal, particularly in the elderly, may occur (see section 4.4). Nausea, vomiting, diarrhoea, flatulence, constipation, dyspepsia, abdominal pain, melaena, haematemesis, ulcerative stomatitis, exacerbation of colitis and Crohn's disease have been reported following its administration (see section 4.4 Special warnings and precautions for use). Gastritis has been observed less frequently. In general, the risk of experiencing adverse reactions (particularly serious gastrointestinal complications) increases with increasing dose and duration of treatment. Hypersensitivity reactions have been reported following treatment with ibuprofen, which may include: (a) non-specific allergic reactions and anaphylaxis; (b) respiratory tract reactivity, including asthma, aggravated asthma, bronchospasm or dyspnoea; (c) various skin disorders, including rashes of various types, pruritus, urticaria, purpura, angioedema and, more rarely, exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme). In patients with active autoimmune disorders (such as systemic lupus erythematosus or mixed connective tissue disease), isolated cases of symptoms of aseptic meningitis, such as stiff neck, headache, nausea, vomiting, fever or disorientation, have been observed during treatment with ibuprofen. Oedema, hypertension and cardiac failure have been reported in association with the use of NSAIDs. Clinical trials suggest that use of ibuprofen, particularly at high doses (2400 mg/day), may be associated with a small increased risk of arterial thrombotic events (for example myocardial infarction or stroke) (see section 4.4). The list of adverse reactions reported below refers to reactions seen with over-the-counter doses of ibuprofen and pseudoephedrine hydrochloride for short-term use. In the treatment of chronic conditions, additional adverse reactions may occur during long-term treatment. Patients should be advised to stop taking Momenxsin immediately and seek medical advice in the event of a serious adverse drug reaction. very common (≥1/10); common (≥1/100, <1/10); uncommon (≥1/1,000, <1/100); rare (≥1/10,000, <1/1,000); very rare (<1/10,000); not known (frequency cannot be estimated from the available data).
| Infections and infestations | Ibuprofen | Very rare | Exacerbation of infectious inflammation (e.g. necrotizing fasciitis), aseptic meningitis (stiff neck, headache, nausea, vomiting, fever or disorientation) in patients with pre-existing autoimmune diseases (systemic lupus erythematosus (SLE), mixed connective tissue disease) |
| Pathologies of the haemolymphopoietic system | Ibuprofen | Very rare | Hematopoietic disorders (anemia, leukopenia, thrombocytopenia, pancytopenia, agranulocytosis) |
| Immune system disorders | Ibuprofen | Uncommon | Hypersensitivity reactions with urticaria, itching and asthma attacks (with drop in blood pressure) |
| Ibuprofen and Pseudoephedrine hydrochloride | Very rare | Severe generalized hypersensitivity reactions, the signs of which may be facial edema, angioedema, dyspnoea, tachycardia, reduction in blood pressure, anaphylactic shock | |
| Psychiatric disorders | Ibuprofen | Very rare | Psychotic reactions, depression |
| Pseudoephedrine hydrochloride | Not known | Agitation, hallucinations, anxiety, abnormal behavior, insomnia | |
| Nervous system disorders | Ibuprofen | Uncommon | Central nervous system disorders, such as headache, dizziness, insomnia, agitation, irritability or tiredness |
| Pseudoephedrine hydrochloride | Rare | Insomnia, nervousness, anxiety, restlessness, tremors, hallucinations | |
| Pseudoephedrine hydrochloride | Not known | Hemorrhagic stroke, ischemic stroke, seizures, headache | |
| Eye pathologies | Ibuprofen | Uncommon | Vision problems |
| Ear and labyrinth pathologies | Ibuprofen | Rare | Tinnitus |
| Heart disease | Ibuprofen | Very rare | Palpitations, heart failure, myocardial infarction |
| Pseudoephedrine hydrochloride | Not known | Palpitations, tachycardia, chest pain, arrhythmia | |
| Vascular pathologies | Ibuprofen | Very rare | High blood pressure |
| Pseudoephedrine hydrochloride | Not known | Hypertension | |
| Respiratory, thoracic and mediastinal pathologies | Pseudoephedrine hydrochloride | Rare | Exacerbation of asthma or hypersensitivity reaction with bronchospasm |
| Gastrointestinal disorders | Ibuprofen | Common | Gastrointestinal discomfort, dyspepsia, abdominal pain, nausea, vomiting, flatulence, diarrhoea, constipation, mild gastrointestinal bleeding which in rare cases leads to anaemia |
| Ibuprofen | Uncommon | Gastrointestinal ulcers in some cases associated with bleeding and/or perforation, gastritis, ulcerative stomatitis, exacerbation of colitis and Crohn's disease (see section 4.4) | |
| Ibuprofen | Very rare | Esophagitis, pancreatitis, diaphragm-like intestinal stenosis | |
| Pseudoephedrine hydrochloride | Not known | Dry mouth, thirst, nausea, vomiting, ischemic colitis | |
| Hepatobiliary pathologies | Ibuprofen | Very rare | Liver dysfunction, liver damage, especially in case of prolonged therapy, liver failure, acute hepatitis |
| Skin and subcutaneous tissue disorders | Ibuprofen | Uncommon | Various skin rashes |
| Ibuprofen | Very rare | Bullous reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (Lyell's syndrome), alopecia, severe skin infections and soft tissue complications in chickenpox infection | |
| Ibuprofen | Not known | Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS Syndrome) Acute Generalized Exanthematous Pustulosis (AGEP) | |
| Pseudoephedrine hydrochloride | Not known | Rash, urticaria, pruritus, hyperhidrosis, severe skin reactions including acute generalized exanthematous pustulosis (AGEP) | |
| Kidney and urinary disorders | Ibuprofen | Rare | Damage to kidney tissue (papillary necrosis) and high concentrations of uric acid in the blood |
| Ibuprofen | Very rare | Increased serum creatinine, edema (particularly in patients with arterial hypertension or renal insufficiency), nephrotic syndrome, interstitial nephritis, acute renal failure | |
| Pseudoephedrine hydrochloride | Not known | Difficulty in urination |
Reporting of suspected adverse reactions: Reporting suspected adverse reactions that occur after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system: at https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse.
OVERDOSE
Overdose Momenxsin 200mg/30mg 12 coated tablets - What are the risks of Momenxsin 200mg/30mg 12 coated tablets in case of overdose?
The clinical effects of overdose are most likely due to the presence of pseudoephedrine hydrochloride in this product, rather than ibuprofen. The effects are not clearly related to the dose taken because of the different sensitivity of different individuals to the sympathomimetic properties. Symptoms due to the sympathomimetic effect CNS depression: e.g. sedation, apnea, cyanosis, coma. CNS stimulation (more likely in children): e.g. insomnia, hallucinations, convulsions, tremors. In addition to the symptoms already mentioned as adverse effects, the following symptoms may occur: hypertensive crisis, cardiac arrhythmias, muscle weakness and tension, euphoria, excitement, thirst, chest pain, dizziness, tinnitus, ataxia, blurred vision, hypotension. Symptoms related to ibuprofen (in addition to the gastrointestinal and neurological symptoms already mentioned as adverse effects) Drowsiness, nystagmus, tinnitus, hypotension, loss of consciousness. In severe poisoning, metabolic acidosis may occur. Therapeutic measures There are no specific antidotes. If the patient presents within one hour of ingestion of a potentially toxic amount of the drug, oral activated charcoal may be considered. Monitoring of electrolytes and performing an ECG are also necessary. In case of cardiovascular instability and/or symptomatic electrolyte imbalance, symptomatic treatment should be initiated.
PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Momenxsin 200mg/30mg 12 coated tablets.
Pregnancy. Pseudoephedrine hydrochloride: Studies in animals have shown reproductive toxicity (see section 5.3). The use of pseudoephedrine hydrochloride reduces maternal uterine blood flow, but clinical data on the effects on pregnancy are insufficient. Ibuprofen : Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryonic/foetal development. Results of epidemiological studies suggest an increased risk of miscarriage, cardiac malformation and gastroschisis following the use of prostaglandin synthesis inhibitors in early pregnancy. The risk is believed to increase proportionally with the dose and duration of therapy. Administration of a prostaglandin synthesis inhibitor to animals has been shown to result in increased pre- and post-implantation loss and embryonic/foetal lethality. In addition, an increased incidence of various malformations, including cardiovascular, has been reported in animals given a prostaglandin synthesis inhibitor during the organogenetic period. During the first and second trimesters of pregnancy, ibuprofen should not be given unless clearly necessary. If a woman attempting to conceive or who is in the first or second trimester of pregnancy must take ibuprofen, the dose and duration of treatment should be kept as low as possible. During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the fetus to: - cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); - renal dysfunction, which may progress to renal failure with oligohydramnios; the mother and child, at the end of pregnancy: - possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses; - inhibition of uterine contractions resulting in delayed or prolonged labor. Consequently, this medicinal product is: contraindicated in the third trimester of pregnancy and should be administered only when strictly necessary in the first and second trimester. Breastfeeding: The need to take measures during breastfeeding arises from the presence of pseudoephedrine hydrochloride in the formulation of the medicinal product: pseudoephedrine hydrochloride is excreted in breast milk. Taking into account the potential cardiovascular and neurological effects of vasoconstrictors, the use of this medicinal product is contraindicated during breastfeeding. Fertility: There is evidence that cyclooxygenase/prostaglandin synthesis inhibitors may impair female fertility by affecting ovulation. The effect is reversible upon discontinuation of treatment.
DRIVING AND USE OF MACHINERY
Taking Momenxsin 200mg/30mg 12 coated tablets before driving or using machines - Does Momenxsin 200mg/30mg 12 coated tablets affect driving or using machines?
Momenxsin slightly or moderately affects the ability to drive or use machines. Patients who experience dizziness, hallucinations, unusual headaches, and vision or hearing disturbances should avoid driving or using machines. Generally, a single administration or the use of this medicine for short periods does not require the adoption of any special precautions.