ANGELINI SpA
Tachipirina Syrup Children Paracetamol 120 mg/5 ml
Tachipirina Syrup Children Paracetamol 120 mg/5 ml

Pickup available at Farmacia Tili
Usually ready in 24 hours
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
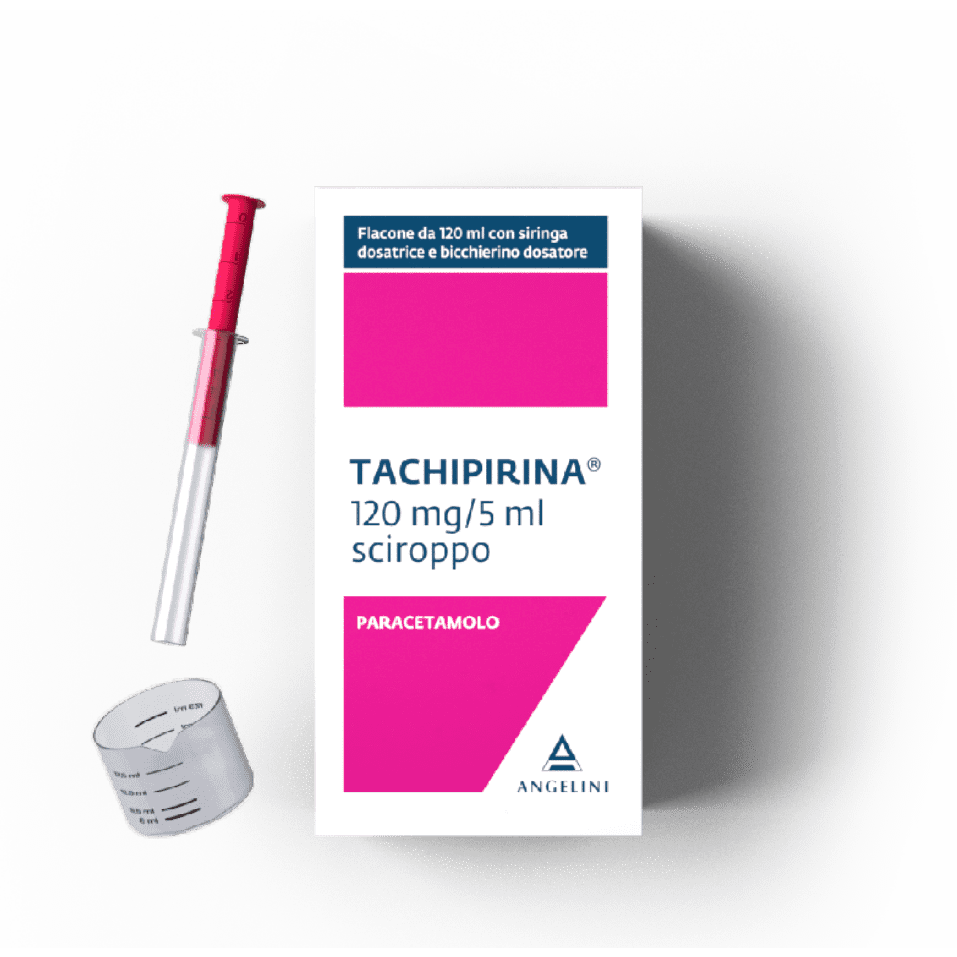
120ml
EAN
EAN
012745016
MINSAN
MINSAN
012745016
Tachipirina Sciroppo 120 mg/5 ml is an over-the-counter medicine based on low-dose Paracetamol for children (from 7.2 kg and up). Paracetamol is preferable in children as an antipyretic to lower temperature and in the symptomatic treatment of febrile conditions such as influenza, exanthematous diseases, acute respiratory tract conditions , etc.
Tachipirina Syrup 120 mg/5 also has an analgesic action in cases of headache , neuralgia , myalgia and other painful manifestations of medium severity and of various origins.
ACTIVE INGREDIENTS
Active ingredients contained in Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - What is the active ingredient of Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml?
TACHIPIRINA 120 mg/5 ml syrup, 5 ml of syrup contain; active ingredient: paracetamol 120 mg. Excipients with known effects: sucrose, methyl parahydroxybenzoate, sodium. TACHIPIRINA 100 mg/ml oral drops solution, 1 ml of solution contains; active ingredient: paracetamol 100 mg. Excipients with known effects: sorbitol, propylene glycol.For the full list of excipients, see section 6.1.
EXCIPIENTS
Composition of Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - What does Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml contain?
Syrup: sucrose, sodium citrate, sodium saccharin, methyl parahydroxybenzoate, potassium sorbate, macrogol 6000, citric acid monohydrate, strawberry flavouring, mandarin flavouring, purified water. Oral drops: propylene glycol, macrogol 6000, sorbitol, sodium saccharin, citrus-vanilla flavouring, propyl gallate, caramel (E150a), sodium edetate, purified water.
DIRECTIONS
Therapeutic indications Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - Why is Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml used? What is it used for?
As an antipyretic: symptomatic treatment of febrile conditions such as influenza, exanthematous diseases, acute respiratory tract conditions, etc. As an analgesic: headaches, neuralgia, myalgia and other moderately severe painful conditions of various origins.
CONTRAINDICATIONS SIDE EFFECTS
Contraindications Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - When should Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml not be used?
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. Patients with severe haemolytic anaemia (this contraindication does not apply to the 500 mg oral formulations). Severe hepatocellular insufficiency (this contraindication does not apply to the 500 mg oral formulations).
DOSAGE
Quantity and method of taking Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - How do you take Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml?
In children up to 10 years of age, it is essential to respect the dosage defined based on body weight and not on age, which is approximate and indicated for information purposes only. If the age of the child does not correspond to the weight shown in the table, always refer to body weight when choosing the dosage. In children weighing up to 7.2 kg, it is recommended to use the formulation in drops, between 7.2 and 11 kg it is possible to use drops or syrup as the dosage for each weight group is identical, between 12 and 32 kg it is recommended to use syrup. In the case of jaundice in children under three months, it is advisable to reduce the single dose. In children over 10 years of age, the relationship between weight and age becomes no longer homogeneous due to pubertal development which, at the same age, has a different impact on body weight depending on the sex and individual characteristics of the child. Therefore, above 10 years of age, the dosage of the syrup is indicated in terms of weight and age ranges, as follows. Children weighing between 33 and 40 kg (over 10 years and under 12 years of age): 20 ml of syrup at a time (corresponding to 480 mg), to be repeated if necessary after 6 hours, without exceeding 4 administrations per day. Adolescents weighing more than 40 kg (aged 12 years or over) and adults: 20 ml of syrup at a time (corresponding to 480 mg), to be repeated if necessary after 4 hours, without exceeding 6 administrations per day. The doctor must evaluate the need for treatments for more than 3 consecutive days. Method of administration The package contains a dosing syringe with level marks corresponding to 1 ml, 2 ml, 3 ml, 4 ml, 4.5 ml and 5 ml and a dosing cup with level marks corresponding to 5.5 ml, 6 ml, 6.5 ml, 7.5 ml, 8.5 ml, 10 ml, 11 ml, 12.5 ml, 13.5 ml, 15.5 ml, 17.5 ml, 19 ml Syrup The syrup contains 24 mg of paracetamol per ml of product. To open the bottle, push the cap downwards and at the same time turn to the left. To use the syringe, insert the tip of the syringe fully into the hole in the undercap, turn the bottle upside down, holding the syringe firmly, gently pull the plunger downwards filling the syringe up to the mark corresponding to the desired dose; Return the bottle to an upright position, remove the syringe by gently rotating it, insert the tip of the syringe into the child's mouth and apply gentle pressure to the plunger to release the solution. The product must be used immediately after taking it from the bottle. Any residual product in the syringe must be discarded. For doses greater than 5 ml, take the required amount with the syringe and pour the contents into the cup. Repeat the procedure until the mark corresponding to the indicated dosage is reached, and administer to the child, inviting him to drink. For doses in children over 10 years of age and in adults, equal to 20 ml, use the cup, filling it twice up to the 10 ml mark. The product must be used immediately after taking it from the bottle. Any residual product in the syringe or cup must be discarded. After use, close the bottle by screwing the cap tightly and wash the syringe and cup with hot water. Leave them to dry, keeping them out of the reach and sight of children. Drops Each drop contains 4 mg of paracetamol. Turn the bottle upside down and pour the number of drops corresponding to the dosage to be used in 25-50 ml of water, and give the child to drink. Renal insufficiency In case of severe renal insufficiency (creatinine clearance less than 10 ml/min), the interval between administrations must be at least 8 hours.
CONSERVATION
Storage Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - How is Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml stored?
This medicinal product does not require any special storage conditions.
WARNINGS
Warnings Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - About Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml it is important to know that:
In rare cases of allergic reactions, administration should be suspended and appropriate treatment should be instituted. Use with caution in cases of chronic alcoholism, excessive alcohol intake (3 or more alcoholic drinks per day), anorexia, bulimia or cachexia, chronic malnutrition (low reserves of hepatic glutathione), dehydration, hypovolemia. Paracetamol should be administered with caution to patients with mild to moderate hepatocellular insufficiency (including Gilbert's syndrome), severe hepatic insufficiency (Child-Pugh>9), acute hepatitis, in concomitant treatment with drugs that alter liver function, glucose-6-phosphate dehydrogenase deficiency, hemolytic anemia. High or prolonged doses of the product can cause alterations to the kidney and blood, even serious, therefore administration to subjects with renal insufficiency should be carried out only if really necessary and under direct medical supervision. In case of prolonged use it is advisable to monitor liver and renal function and blood count. During treatment with paracetamol, before taking any other medicine, check that it does not contain the same active ingredient, since if paracetamol is taken in high doses, serious adverse reactions may occur. Invite the patient to contact the doctor before combining any other medicine (see section 4.5). Important information about some of the excipients. Tachipirina drops, solution contains, sorbitol: patients with rare hereditary problems of fructose intolerance, should not take this medicine; propylene glycol: may cause symptoms similar to those caused by alcohol. The container of Tachipirina drops, solution is made of latex rubber. May cause serious allergic reactions. Tachipirina syrup contains, sucrose: patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase insufficiency, should not take this medicine. For the 15 ml dose this medicinal product contains 5.25 g sucrose, for the 16.5 ml dose it contains 5.78 g sucrose, for the 18.5 ml dose it contains 6.48 g sucrose and for the 20 ml dose it contains 7 g sucrose. To be taken into consideration by people with diabetes mellitus. Methyl parahydroxybenzoate: may cause allergic reactions (possibly delayed). Sodium: this medicinal product contains 1.2 mmol (or 27.6 mg) sodium per 20 ml equivalent to 1.38% of the WHO recommended maximum daily intake of 2 g sodium for an adult. To be taken into consideration by people on a controlled sodium diet.
INTERACTIONS
Interactions Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml - Which medicines or foods can modify the effect of Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml?'
The oral absorption of paracetamol depends on the rate of gastric emptying. Therefore, the concomitant administration of drugs that slow (e.g. anticholinergics, opioids) or increase (e.g. prokinetics) the rate of gastric emptying may determine a decrease or increase respectively in the bioavailability of the product. The concomitant administration of cholestyramine reduces the absorption of paracetamol. The simultaneous intake of paracetamol and chloramphenicol may induce an increase in the half-life of chloramphenicol, with the risk of increasing toxicity. Concomitant use of paracetamol (4 g daily for at least 4 days) with oral anticoagulants may cause slight variations in INR values. In these cases, more frequent monitoring of INR values should be performed during concomitant use and after its interruption. Use with extreme caution and under close supervision during chronic treatment with drugs that can determine the induction of hepatic monooxygenases or in case of exposure to substances that can have this effect (for example rifampicin, cimetidine, antiepileptics such as glutethimide, phenobarbital, carbamazepine). The same applies in cases of alcoholism and in patients treated with zidovudine. The administration of paracetamol may interfere with the determination of uricemia (by the phosphotungstic acid method) and with that of glycemia (by the glucose-oxidase-peroxidase method).
SIDE EFFECTS
Like all medicines, Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml can cause side effects - What are the side effects of Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml?
The following are the adverse reactions of paracetamol organised according to the MedDRA system organ classification. There are insufficient data to establish the frequency of the individual effects listed. Blood and lymphatic system disorders: thrombocytopenia, leukopenia, anaemia, agranulocytosis. Immune system disorders: hypersensitivity reactions (urticaria, laryngeal edema, angioedema, anaphylactic shock). Nervous system disorders: dizziness. Gastrointestinal disorders: gastrointestinal reaction. Hepatobiliary disorders: abnormal liver function, hepatitis. Skin and subcutaneous tissue disorders: erythema multiforme, Stevens Johnson syndrome, epidermal necrolysis, rash. Renal and urinary disorders: acute renal failure, interstitial nephritis, haematuria, anuria. Very rare cases of serious skin reactions have been reported. Reporting of suspected adverse reactions. Reporting of suspected adverse reactions that occur after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system at https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse.
PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Tachipirina Sciroppo Bambini Paracetamol 120 mg/5 ml
Pregnancy: A large amount of data on pregnant women indicate neither malformative nor fetal/neonatal toxicity. Epidemiological studies on neurodevelopment in children exposed to paracetamol in utero show inconclusive results. If clinically necessary, paracetamol can be used during pregnancy, however it should be used at the lowest effective dose for the shortest possible time and with the lowest possible frequency. Breastfeeding: It is recommended to take/administer this medicine only in cases of real need and under direct medical supervision.