PROCTER & GAMBLE SRL
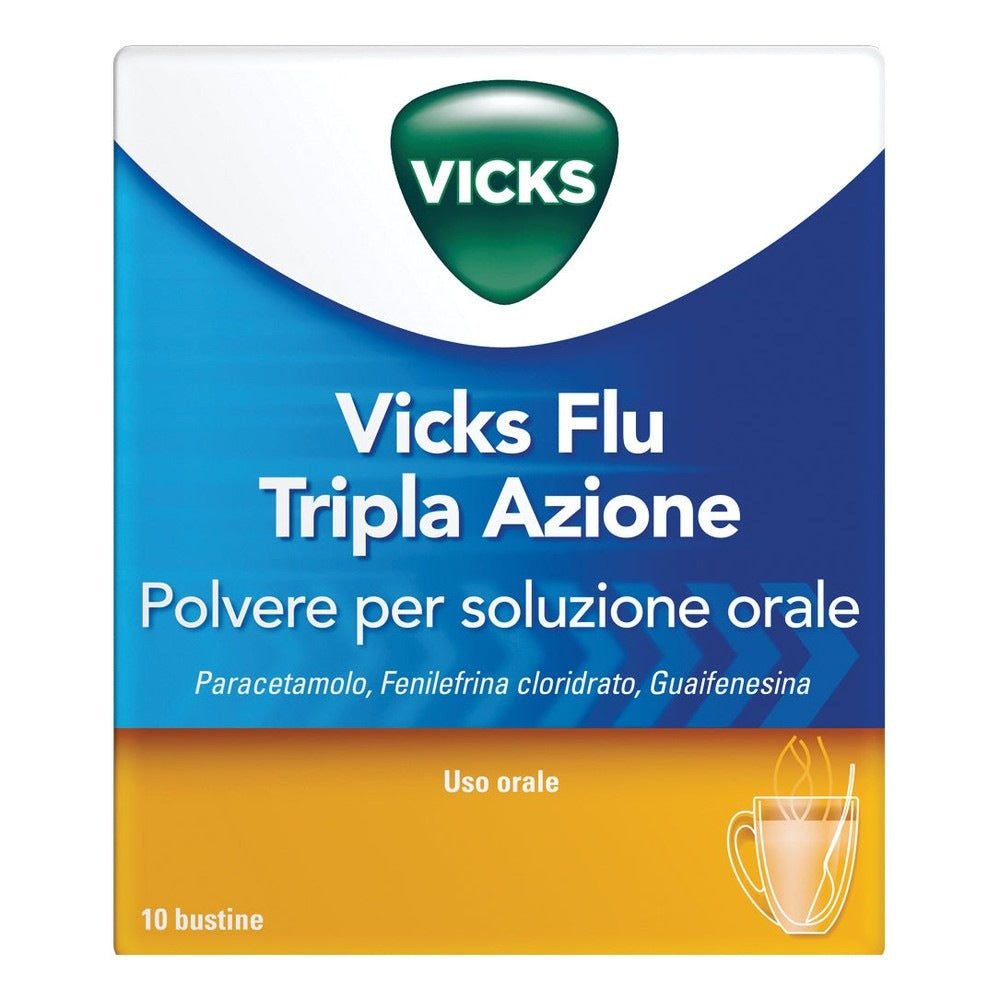
Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution
Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution
Regular price
€10,14
Regular price
€0,00
Sale price
€10,14
Unit price
per
Tax included.
Shipping calculated at checkout.

Pickup available at Farmacia Tili
Usually ready in 24 hours
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
EAN
EAN
039773027
MINSAN
MINSAN
039773027
Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution is an effective treatment for the short-term symptomatic relief of conditions such as mild to moderate pain , fever , nasal congestion and productive cough associated with colds, chills and flu. Each sachet contains a combination of 500mg paracetamol , 200mg guaifenesin and 10mg phenylephrine hydrochloride , which work synergistically to relieve flu symptoms. The powder formulation is designed to be dissolved in hot water, offering a quick and convenient option for those looking for immediate relief. With the addition of lemon flavouring, the product is not only effective, but also pleasant to take. Vicks Flu Triple Action is ideal for adults and children aged 12 years and over, providing comprehensive support against flu symptoms.
ACTIVE INGREDIENTS
Active ingredients contained in Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - What is the active ingredient in Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution?
One sachet contains: 500 mg paracetamol 200 mg guaifenesin 10 mg phenylephrine hydrochloride Excipients: Sucrose 2000 mg Aspartame 6 mg Sodium 157 mg For the full list of excipients, see section 6.1.EXCIPIENTS
Composition of Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - What does Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution contain?
Sucrose Citric acid Tartaric acid Sodium cyclamate Sodium citrate Aspartame (E951) Acesulfame potassium (E950) Menthol powder Lemon flavour Lemon juice flavour Quinoline yellow (E104)DIRECTIONS
Therapeutic indications Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - Why is Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution used? What is it for?
Short-term symptomatic treatment of mild to moderate pain, fever, nasal congestion with expectorant effect in case of productive cough, associated with cold, chills and influenza.CONTRAINDICATIONS SIDE EFFECTS
Contraindications Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - When should Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution not be used?
Hypersensitivity to paracetamol, guaifenesin, phenylephrine hydrochloride or to any of the excipients. Hepatic impairment or severe renal impairment Hypertension Hyperthyroidism Diabetes Cardiac disease. Angle-closure glaucoma Porphyria Use in patients who are taking tricyclic antidepressants Use in patients who are taking, or who have taken in the previous 2 weeks, monoamine oxidase inhibitors (MAOIs). Use in patients who are taking beta-blockers Use in patients who are taking other sympathomimetic drugs Children under 12 years.DOSAGE
Quantity and method of taking Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - How do you take Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution?
Dissolve the contents of one sachet in a medium-sized cup and add hot, not boiling, water (approximately 250 ml). Allow to cool to a drinkable temperature. Adults and children aged 12 years and over: one sachet. Repeat every four hours as directed, without exceeding four doses (sachets) in any 24-hour period. Do not administer to children under 12 years of age without medical advice. Do not administer to patients with hepatic impairment or severe renal impairment (see section 4.3). Consult your doctor if symptoms persist for more than 3 days.CONSERVATION
Storage Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - How do you store Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution?
Do not store above 25°C.WARNINGS
Warnings Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - About Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution it is important to know that:
Prolonged use of the product is not recommended. Patients should be advised not to take other products containing paracetamol or containing the same active ingredients as this preparation. Patients should also be advised to avoid taking alcohol, other decongestant products or products against cough or cold at the same time. The doctor or pharmacist must check that preparations containing sympathomimetics are not administered simultaneously through multiple routes, i.e. orally and topically (nasal, auricular and ocular preparations). This medicinal product should be recommended only in the presence of all symptoms (pain and/or fever, nasal congestion and bronchial cough). The risks associated with overdose are greater in patients with non-cirrhotic alcoholic liver disease. Use with caution in patients taking digitalis, beta-adrenergic blockers, methyldopa or other antihypertensive agents (see section 4.5). Use with caution in patients with prostatic hypertrophy as they are potentially subject to urinary retention. Sympathomimetic-containing products should be used with great care in patients who are taking phenothiazines. Use in patients with Raynaud's phenomenon. Consult your doctor before use in case of persistent or chronic cough such as that caused by smoking, asthma, chronic bronchitis or emphysema. Contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. Contains 157 mg sodium per dose. To be taken into consideration by patients with reduced kidney function or on a controlled sodium diet. Contains aspartame (E951). This medicine contains a source of phenylalanine. May be harmful if you have phenylketonuria.INTERACTIONS
Interactions Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - Which medicines or foods can modify the effect of Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution?
The hepatotoxicity of paracetamol may be potentiated by excessive alcohol intake. The rate of absorption of paracetamol may be increased with metoclopramide or domperidone and the absorption reduced with cholestyramine. Substances that induce hepatic microsomal enzymes, such as alcohol, barbiturates, monoamine oxidase inhibitors and tricyclic antidepressants, may increase the hepatotoxicity of paracetamol, particularly following overdose. Isoniazid reduces the elimination of paracetamol, with possible potentiation of its activity and/or toxicity, by inhibiting its metabolism in the liver. Probenecid causes a halving of the elimination of paracetamol, by inhibiting its binding to glucuronic acid. A reduction of paracetamol should be considered if probenecid is being taken. Regular use of paracetamol may reduce the metabolism of Zidovudine (increasing the risk of neutropenia). Interactions between sympathomimetic amines, such as phenylephrine, and monoamine oxidase inhibitors cause hypertensive effects. Phenylephrine may interact negatively with sympathomimetic agents and may reduce the efficacy of beta-blockers, methyldopa and other antihypertensive drugs (see section 4.4). The conditions in which these drugs are taken constitute contraindications to the use of the product. The anticoagulant effect of warfarin and other coumarin drugs may be potentiated by regular and prolonged intake of paracetamol, with an increased risk of haemorrhages; occasional doses have no significant effect. Drug interactions between paracetamol and various other drugs have been reported. It is considered unlikely that these interactions are clinically significant in temporary use and in accordance with the suggested dosage regimen. Salicylates/aspirin may prolong the elimination (t ½) of paracetamol. Paracetamol may decrease the bioequivalence of lamotrigine, with possible decrease in its effect, due to a possible increase in its metabolism in the liver. It is possible that digitalis may sensitize the myocardium to effects similar to sympathomimetic substances. Paracetamol may alter the phosphotungstate uric acid test and the blood glucose test.SIDE EFFECTS
Like all medicines, Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution can cause side effects - What are the side effects of Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution?
The incidence of adverse reactions is usually classified as follows: Very common (>10) Common (>1/100 to <1/10) Uncommon (>1/1000 to <1/100) Rare (>1/10,000 to < 1/1000) Very rare (<1/10,000) Not known (incidence cannot be classified based on the available data) Cardiac disorders: Phenylephrine may rarely be associated with tachycardia (≥1/10,000 to ≤1 in 1000). Blood and lymphatic system disorders: Very rarely (<1 in 10,000), blood dyscrasias such as thrombocytopenia, agranulocytosis, haemolytic anaemia, neutropenia, leucopenia and pancytopenia have been reported following paracetamol intake, although there may be no causal relationship. Nervous system disorders: As with other sympathomimetic amines, insomnia, nervousness, tremor, anxiety, restlessness, confusion, irritability and headache may occur rarely (≥1/10,000 to ≤1 in 1000). Guaifenesin is also known to cause headache and dizziness rarely (≥1/10,000 to ≤1 in 1000). Gastrointestinal disorders: Anorexia, nausea and vomiting are common with sympathomimetics (≥1 in 100 to ≤1 in 10) and may occur with phenylephrine. Gastrointestinal disturbances, nausea, vomiting and diarrhoea are the most common adverse effects associated with guaifenesin, but occur rarely (≥1/10,000 to ≤1 in 1,000). Gastrointestinal effects of paracetamol are very rare but cases of acute pancreatitis have been reported following ingestion of higher than normal doses. Renal and urinary disorders: Interstitial nephritis has been reported incidentally after prolonged use of high doses of paracetamol. Skin and subcutaneous tissue disorders: Hypersensitivity, including skin rash, and urticaria (≥1/10,000 to ≤1 in 1,000) may rarely occur following the intake of paracetamol. Vascular disorders: Increased blood pressure associated with headache, vomiting and palpitations may rarely occur following the intake of phenylephrine (≥1/10,000 to ≤1 in 1,000). Immune system disorders: Rare cases (≥1/10,000 to ≤1 in 1,000) of allergic or hypersensitivity reactions have been reported following the intake of both phenylephrine and paracetamol, including skin rash, urticaria, anaphylaxis and bronchospasm. Hepatobiliary disorders: rarely (≥ 1/10,000 to ≤ 1/1000), abnormalities in liver function tests (increased liver transaminases).OVERDOSE
Overdose Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution - What are the risks of Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution in case of overdose?
PARACETAMOL There is a risk of poisoning particularly in elderly patients, young children, patients with liver disease, chronic alcoholics, patients with chronic malnutrition. In these cases, an overdose can be fatal. In adults, a quantity of paracetamol equal to or greater than 10 g can cause liver damage. If the patient has one of the risk factors (see below), ingestion of a quantity of paracetamol equal to or greater than 5 g can cause liver damage. Risk factors If the patient: a) is undergoing prolonged therapy with carbamazepine, phenobarbital, phenytoin, primidone, rifampicin, St. John's Wort or other drugs that induce liver enzymes or b) regularly consumes ethanol in excess of the recommended quantities or c) has a glutathione deficiency, e.g. in case of eating disorders, cystic fibrosis, HIV infection, starvation, cachexia. Symptoms Symptoms of paracetamol overdose that occur within the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may occur within 12–48 hours of ingestion. Abnormalities in glucose metabolism and metabolic acidosis may occur. In cases of severe poisoning, liver failure may progress to encephalopathy, haemorrhage, hypoglycemia, cerebral oedema and death. Even in the absence of severe liver damage, acute renal failure with acute tubular necrosis may occur, which is highly likely if accompanied by back pain, haematuria and proteinuria. Cases of cardiac arrhythmia and pancreatitis have been reported. Treatment In the event of paracetamol overdose, it is essential to treat the patient immediately. Even in the absence of significant initial symptoms, the patient should be transferred to hospital urgently. Symptoms may be limited to nausea or vomiting and may not reflect the severity of the overdose or the risk of organ damage. Treatment of the patient should be carried out in accordance with current guidelines. If less than 1 hour has elapsed since the overdose, treatment with activated charcoal may be considered. Plasma paracetamol concentrations should be measured no earlier than 4 hours after ingestion (plasma concentrations measured earlier are unreliable). Within 24 hours of ingestion of paracetamol, the patient may be treated with N-acetylcysteine; however, the maximum protective effect is obtained within 8 hours of ingestion. After this time, the efficacy of the antidote decreases dramatically. If necessary, the patient should be given N-acetylcysteine intravenously, in line with the established dosing schedule. If vomiting is not a problem and the patient is away from hospital, oral methionine may be a valid alternative. Treatment of patients with severe hepatic dysfunction presenting more than 24 hours after ingestion should be discussed with the poison control center or a hepatology department. PHENYLEPHRINE HCL Symptoms of phenylephrine overdose include irritability, headache, hypertension, reflex bradycardia and arrhythmias. Hypertension should be treated with an alpha-blocker such as phentolamine intravenously. Reduction in blood pressure may reflexively increase heart rate but, if necessary, this can be facilitated by atropine. GUAIFENESIN Mild to moderate overdose may cause dizziness and gastrointestinal disturbances. Very large doses may produce excitation, confusion and respiratory depression. Urinary calculi have been reported in patients consuming large quantities of guaifenesin-containing preparations. Treatment is symptomatic and includes gastric lavage and general supportive measures.PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution.
Epidemiological studies in pregnant women have shown no adverse effects due to the use of paracetamol in the recommended doses; however, pregnant patients should follow their doctor's instructions regarding the use of the drug. Paracetamol is excreted in breast milk, although not in clinically significant quantities. Available published data do not report contraindications regarding breastfeeding. Data on the use of phenylephrine during pregnancy are limited. Vasoconstriction of the uterine vessels and reduction of uterine blood flow associated with the use of phenylephrine may cause fetal hypoxia. Until further information is available, the use of phenylephrine during pregnancy should be avoided, except in cases where the doctor considers it essential. There are no data available regarding the possible release of phenylephrine into breast milk nor are there reports regarding the effects of phenylephrine on breast-fed infants. Until further data are available, the use of phenylephrine during breastfeeding should be avoided, except when the doctor considers it essential. The safety of guaifenesin during pregnancy and breastfeeding has not yet been fully established. During pregnancy, the product should be taken only when the doctor considers it essential.DRIVING AND USE OF MACHINERY
Taking Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution before driving or using machines - Does Vicks Flu Triple Action 500mg/200mg/10mg 10 sachets powder for oral solution affect driving or using machines?
No studies on the ability to drive and use machines have been performed. When performing these activities, the possibility of adverse events, such as dizziness and confusion, should be considered.