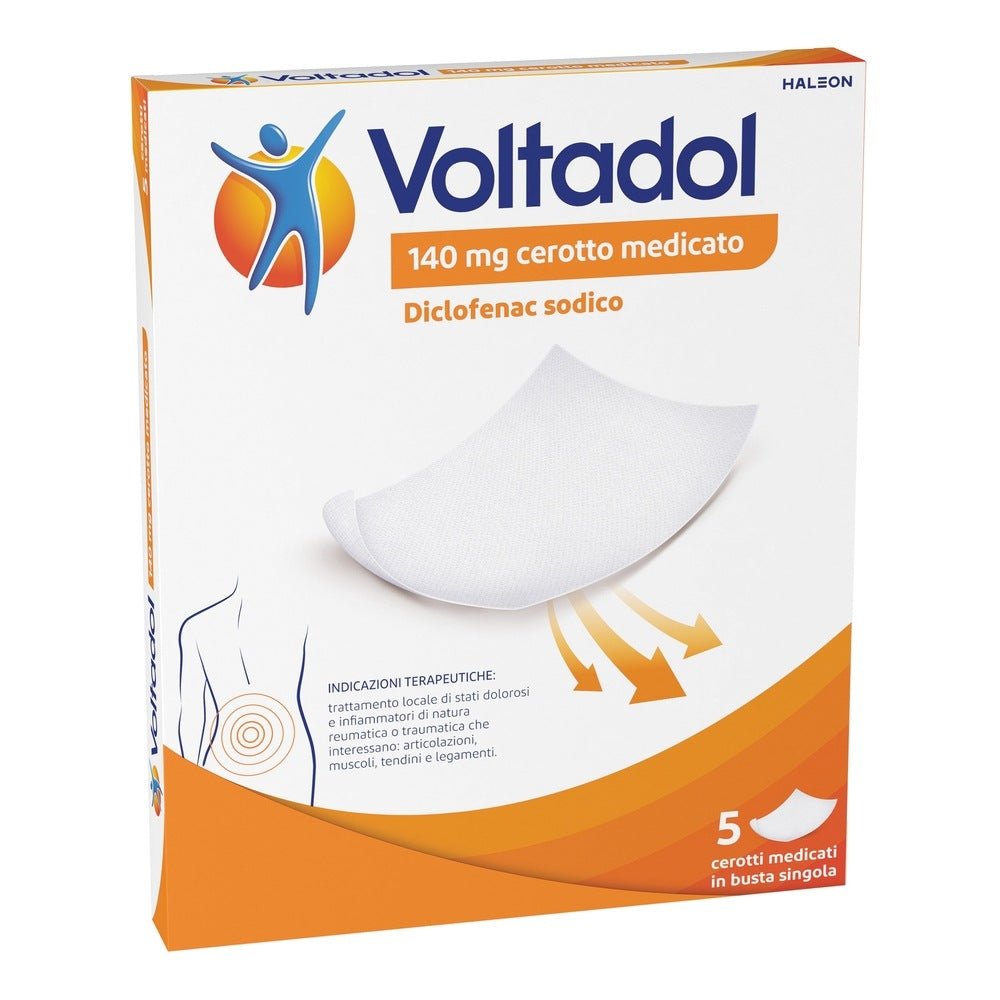
HALEON ITALY Srl
Voltadol 140mg 5 medicated plasters
Voltadol 140mg 5 medicated plasters
Regular price
€16,95
Regular price
€16,95
Sale price
€16,95
Unit price
per
Tax included.
Shipping calculated at checkout.

Couldn't load pickup availability
PRODUCT NET WEIGHT
PRODUCT NET WEIGHT
EAN
EAN
035520016
MINSAN
MINSAN
035520016
Voltadol 140mg 5 medicated plasters is an effective topical treatment for the local relief of pain and inflammation of rheumatic or traumatic origin involving joints , muscles , tendons and ligaments . Each plaster contains diclofenac sodium 140 mg , an active ingredient known for its anti-inflammatory and analgesic properties. Designed for direct application to the skin, Voltadol offers a targeted release of the active ingredient, ensuring prolonged and localized action. Ideal for those looking for a practical and non-invasive alternative to oral treatments, Voltadol stands out for its ease of use and effectiveness in reducing pain and inflammation.
Reporting of suspected adverse reactions. Reporting suspected adverse reactions that occur after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system at https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse
ACTIVE INGREDIENTS
Active ingredients contained in Voltadol 140mg 5 medicated patches - What is the active ingredient in Voltadol 140mg 5 medicated patches?
One medicated plaster contains: active substance: diclofenac sodium 140 mg. For the full list of excipients, see section 6.1.EXCIPIENTS
Composition of Voltadol 140mg 5 medicated plasters - What does Voltadol 140mg 5 medicated plasters contain?
Butyl methacrylate copolymer basic; acrylate-vinyl acetate copolymer; polyethylene glycol 12 stearate; sorbitan oleate; non-woven fabric; siliconized paper.DIRECTIONS
Therapeutic indications Voltadol 140mg 5 medicated plasters - Why is Voltadol 140mg 5 medicated plasters used? What is it used for?
Local treatment of painful and inflammatory conditions of rheumatic or traumatic nature of the joints, muscles, tendons and ligaments.CONTRAINDICATIONS SIDE EFFECTS
Contraindications Voltadol 140mg 5 medicated patches - When should Voltadol 140mg 5 medicated patches not be used?
Hypersensitivity to the active substance, to acetylsalicylic acid or to other nonsteroidal anti-inflammatory drugs (NSAIDs) or to any of the excipients listed in section 6.1. Patients with a history of asthma, angioedema, urticaria or acute rhinitis after taking acetylsalicylic acid or other nonsteroidal anti-inflammatory drugs (NSAIDs). Damaged skin, regardless of the type of lesion: exudative dermatitis, eczema, infected lesion, burns or wounds. Third trimester of pregnancy (see section 4.6). Patients with active peptic ulcer. Children and adolescents under 16 years of age.DOSAGE
Quantity and method of taking Voltadol 140mg 5 medicated patches - How to take Voltadol 140mg 5 medicated patches?
For cutaneous use only. Dosage VOLTADOL should only be applied to intact, healthy skin and should not be applied while bathing or showering. VOLTADOL should be used for the shortest possible time. Adults and adolescents aged 16 years and over: Apply one patch twice a day, in the morning and in the evening, to the skin of the area to be treated, for a period not exceeding 7 days. Do not exceed the recommended dose. If no improvement is seen or if worsening of symptoms is reported after 7 days of treatment, the situation should be reassessed (see section 4.4). Special populations Children and adolescents under 16 years: VOLTADOL is contraindicated in children and adolescents under 16 years of age (see section 4.3). Elderly and patients with hepatic or renal insufficiency VOLTADOL should be used with caution (see section 4.4). Method of administration 1 - Cut the sachet along the dotted line and remove the patch. To apply the patch : 2 - Remove one of the two protective sheets. 3 - Apply to the area to be treated and remove the remaining protective sheet. 4 - Apply light pressure with the palm of your hand until it adheres completely to the skin. The patch must be used whole. To remove the patch : 5 - Wet the patch with water and then lift a flap, pulling gently. 6 - To eliminate any residue of the product, wash the affected area with water, making circular movements with your fingers.CONSERVATION
Storage Voltadol 140mg 5 medicated plasters - How to store Voltadol 140mg 5 medicated plasters?
Do not store above 30°C.WARNINGS
Warnings Voltadol 140mg 5 medicated patches - About Voltadol 140mg 5 medicated patches it is important to know that:
If VOLTADOL is used for a prolonged period of time, the possibility of systemic adverse events cannot be excluded. VOLTADOL should only be applied to intact, healthy skin and should not be applied to broken skin or open wounds. The plasters should not come into contact with the eyes or mucous membranes and should not be swallowed. Undesirable effects can be minimised by using the lowest effective dose for the shortest duration necessary to control symptoms. Treatment should be stopped immediately if a skin rash develops after application of the medicated plaster. Patients with asthma, chronic obstructive bronchial disease, allergic rhinitis or inflammation of the nasal mucosa (nasal polyp) react to treatment with NSAIDs more often than other patients with asthma attacks, local inflammation of the skin or mucosa (Quincke's oedema) or urticaria. Administration of VOLTADOL should be discontinued in women who have fertility problems or who are undergoing investigation of fertility. The use, especially if prolonged, of products for topical use may give rise to sensitization phenomena. In this case it is necessary to interrupt the treatment and start an appropriate therapy. Although systemic absorption is minimal, the use of VOLTADOL is not recommended in women who intend to become pregnant. Do not administer another medicinal product containing diclofenac or other NSAIDs topically or systemically at the same time. The use of VOLTADOL in association with other medicinal products containing diclofenac may give rise to skin reactions with serious evolution (Stevens-Johnson syndrome, Lyell syndrome). Given the route of administration, the risk of onset of systemic effects is lower, however the medicated plaster must be used with caution in patients with renal, cardiac or hepatic impairment, history of peptic ulcer or inflammatory bowel disease or haemorrhagic diathesis. NSAIDs must be used with particular caution in elderly patients who are more predisposed to undesirable effects. Topical diclofenac may be used with non-occlusive dressings, but should not be used with an occlusive dressing that does not allow air to pass through. Patients should be advised not to expose themselves to direct sunlight or sunlamps for approximately one day after removal of the medicated plaster in order to reduce the risk of photosensitivity.INTERACTIONS
Interactions Voltadol 140mg 5 medicated patches - Which medicines or foods can modify the effect of Voltadol 140mg 5 medicated patches?
The systemic absorption of diclofenac following the use of medicated plasters is low. However, the possibility of competition between absorbed diclofenac and other drugs with high plasma protein binding cannot be excluded. Concomitant topical or systemic use of other drugs containing diclofenac or other NSAIDs is not recommended.SIDE EFFECTS
Like all medicines, Voltadol 140mg 5 medicated patches can cause side effects - What are the side effects of Voltadol 140mg 5 medicated patches?
Adverse reactions (Table 1) are listed by frequency, the most frequent first, using the following convention: common (≥ 1/100, <1/10); uncommon (≥1/1,000, <1/100); rare (≥1/10,000, <1/1,000); very rare (<1/10,000); not known: frequency cannot be estimated from the available data. Table 1| Infections and infestations | |
| Very rare: | Rash with pustules |
| Immune system disorders | |
| Very rare: | Hypersensitivity (including urticaria), angioneurotic edema, anaphylactoid reaction |
| Respiratory, thoracic and mediastinal pathologies | |
| Very rare: | Asthma |
| Skin and subcutaneous tissue disorders | |
| Common: | Rash, eczema, erythema, dermatitis (including allergic dermatitis and contact dermatitis), pruritus |
| Rare: | Bullous dermatitis (e.g. erythema bullosum), burning sensation at the application site, dry skin |
| Very rare: | Photosensitivity reaction |
| Systemic disorders and conditions related to the administration site | |
| Common: | Reactions at the administration site |
OVERDOSE
Voltadol 140mg 5 medicated patches Overdose - What are the risks of Voltadol 140mg 5 medicated patches in case of overdose?
Cases of overdose with VOLTADOL have been reported, but no systemic adverse effects that may be caused by overdose with oral NSAIDs (e.g. vomiting, diarrhoea, dizziness, tinnitus, gastrointestinal bleeding, convulsions) have been reported. However, adverse reactions similar to those observed after an overdose of diclofenac tablets may be expected if topical diclofenac is inadvertently ingested (1 pack of 10 patches contains 1400 mg diclofenac sodium). If systemic adverse reactions occur due to incorrect use or accidental overdose (e.g. in children) with the product, the general supportive therapeutic measures to be taken in the case of intoxication with non-steroidal anti-inflammatory drugs are recommended. Further treatment modalities should take into account the recommendations of the poison control centre, where available.PREGNANCY AND BREASTFEEDING
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking Voltadol 140mg 5 medicated plasters.
Pregnancy The systemic concentration of diclofenac compared to oral formulations is lower after topical administration. Based on experience with treatment with systemic NSAIDs, the following is recommended: Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/fetal development. Results of epidemiological studies suggest an increased risk of miscarriage and of cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk of cardiac malformations increased from less than 1%, up to approximately 1.5%. The risk was believed to increase with dose and duration of therapy. In animals, administration of prostaglandin synthesis inhibitors has been shown to result in increased pre- and post-implantation loss and embryo-fetal mortality. In addition, an increased incidence of various malformations, including cardiovascular, has been reported in animals given prostaglandin synthesis inhibitors during the organogenetic period. During the first and second trimester of pregnancy, diclofenac should not be given unless clearly necessary. If diclofenac is used by a woman attempting to conceive, or during the first and second trimester of pregnancy, the dose should be kept as low and duration of treatment as short as possible. During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the fetus to: - cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); - renal dysfunction, which may progress to renal failure with oligo-hydroamniosis. The mother and the neonate, at the end of pregnancy, to: - possible prolongation of bleeding time, and anti-aggregating effect which may occur even at very low doses; - inhibition of uterine contractions resulting in delayed or prolonged labor. Consequently, diclofenac is contraindicated during the third trimester of pregnancy. Breastfeeding Like other NSAIDs, diclofenac passes into breast milk in small quantities. However, at therapeutic doses of VOLTADOL no effects on the infant are anticipated. Due to the lack of controlled studies in breast-feeding women, the administration of VOLTADOL during breast-feeding should be considered only if the expected benefit to the mother outweighs the risk to the child. In this circumstance, Voltadol should not be applied to the breasts of nursing mothers, nor for a prolonged period of time (see section 4.4).DRIVING AND USE OF MACHINERY
Taking Voltadol 140mg 5 medicated patches before driving or using machines - Does Voltadol 140mg 5 medicated patches affect driving or using machines?
VOLTADOL does not alter the ability to drive vehicles or use machines.